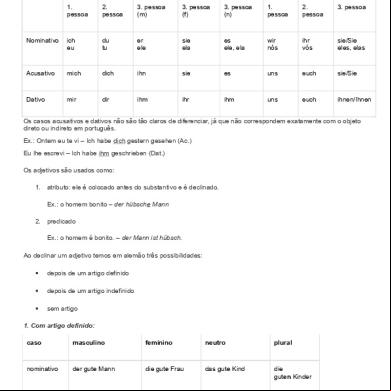
Protein Gel Electrophoresis Technical Handbook 114311
This document was ed by and they confirmed that they have the permission to share it. If you are author or own the copyright of this book, please report to us by using this report form. Report 445h4w
Overview 1s532p
& View Protein Gel Electrophoresis Technical Handbook as PDF for free.
More details 6h715l
- Words: 28,044
- Pages: 45
Western blotting
Protein gel electrophoresis technical handbook separate transfer detect
2
Select precast gel
Comprehensive solutions designed to drive your success
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Post stain
Contents Electrophoresis overview
4
Select precast gel
Protein gel electrophoresis is a simple way to separate proteins prior to downstream detection or analysis, and is a critical step in most workflows that isolate, identify, and characterize proteins. We offer a complete array of products to rapid, reliable protein electrophoresis for a variety of applications, whether it is the first or last step in your workflow. Our portfolio of high-quality protein electrophoresis products unites gels, stains, molecular weight markers, running buffers, and blotting products for your experiments.
8 10
Gel selection guide Gels
Prepare samples and select buffers Sample prep kits 26 Buffers and reagents 28 Buffers and reagents selection guide 29
Select the standard Protein ladders 34 Protein standards selection guide 36
Choose the electrophoresis chamber system and power supply Electrophoresis chamber systems 50 Electrophoresis chamber system selection guide 51 Power supplies 58
Run the gel Gel run conditions 59 Troubleshooting tips 60
Stain the gel Protein stains 62 Protein stains selection guides 63, 67, 69, 70 Electrophoretic staining technology 71
Post stain Transfer and detection 74
Appendix Protocol quick reference 76 Ordering information 81
For a complete listing of all available products and more, visit thermofisher.com/separate
For ordering information refer to page XX. For quick reference on the protocol please refer to page XX.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
3
4
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Post stain
Electrophoresis matrix Electrophoresis is defined as the Two types of matrices are commonly used in transport of charged molecules electrophoresis—polyacrylamide and agarose. The matrices act as porous media and behave like a molecular sieve. through a solvent by an electric Separation of molecules is dependent upon the gel pore size of the matrix used. Agarose has a large pore size and is ideal field. Electrophoresis is a simple, for separating macromolecules such as nucleic acids and protein complexes. Polyacrylamide has a smaller pore size and is ideal for rapid, and sensitive analytical separating most proteins and smaller nucleic acids. tool for separating proteins and Polyacrylamide gel electrophoresis nucleic acids. Any charged ion or (PAGE) molecule will migrate when placed Polyacrylamide gels are generated by the polymerization of acrylamide monomers. These monomers are crosslinked into in an electric field. Most biological long chains by the addition of bifunctional compounds such as (bis), which react with the free molecules carry a net charge at any N,N,-methylenebisacrylamide functional groups of the chain termini. The concentration of acrylamide and bisacrylamide determines the pore size of the gel. pH other than at their isoelectric The higher the acrylamide concentration, the smaller the pore size, resulting in resolution of lower molecular weight molecules and point and will migrate at a rate vice versa. proportional to their charge density. PAGE allows one to separate proteins for different applications
The mobility of a biological molecule through an electric field will depend on the following factors:
based on: • The acrylamide matrix • Buffer systems
Electrophoresis conditions
Linear vs. gradient gels
The separation of molecules is dependent on the electrophoresis conditions. Electrophoresis can be performed under the following conditions:
Gels that have a single acrylamide percentage are referred to as linear gels, and those with a range are referred to as gradient gels. The advantage of using a gradient gel is that it allows the separation of a broader range of proteins than a linear gel.
Continuous vs. discontinuous gels Researchers occasionally refer to gels as continuous or discontinuous. A continuous gel is a gel that has been formed from a single acrylamide solution in the entire gel cassette. A discontinuous gel is formed from two acrylamide solutions, a small, low-percentage stacking gel where the protein wells reside, and a larger portion of gel that separates the proteins. In the traditional Tris-glycine protein gel system, the proteins are stacked in the stacking gel between the highly mobile leading chloride ions (in the gel buffer) and the slower, trailing glycine ions (in the running buffer). The reason for using the stacking gel is to improve the resolution of the bands in the gel. These stacked protein bands undergo sieving once they reach the separating gel.
Mini vs. midi protein gels Commercial gels are available in two size formats, minigels and midigels. Both gels have similar run lengths, but midigels are wider than minigels, allowing midigels to have more wells or larger wells. The additional wells in the midigels permit more samples or large sample volumes to be loaded onto one gel.
Denaturing conditions Electrophoresis is performed under denaturing conditions using an anionic detergent such as sodium dodecylsulfate (SDS). SDS denatures and unfolds the protein by wrapping around the hydrophobic portions. SDS binds at a ratio of ~1.4 g SDS per gram of protein. The resultant SDS-protein complexes are highly negatively charged and are resolved in the gel based on their size.
Nondenaturing (native) conditions Electrophoresis is performed under nondenaturing (native) conditions using buffer systems that maintain the native protein conformation, subunit interaction, and biological activity. During native electrophoresis, proteins are separated based on their charge to mass ratios.
Reducing conditions Electrophoresis is performed under reducing conditions using reducing agents such as dithiothreitol (DTT), β-mercaptoethanol (β-ME) or tris(2-carboxyethyl)phosphine (TCEP). The reducing agents completely unfold the denatured proteins into their subunits by cleaving the disulfide bonds between cysteine residues.
Buffer systems
• Electrophoresis conditions
Electrophoresis is performed using continuous or discontinuous buffer systems. A continuous buffer system utilizes only one buffer in the gel and running buffer. A discontinuous buffer system utilizes a different gel buffer and running buffer1. This system may also use two gel layers of different pore sizes and different buffer composition (the stacking and separating gel). Electrophoresis using a discontinuous buffer system results in concentration of the sample and higher resolution.
• Field strength • Net charge on the molecule • Size and shape of the molecule • Ionic strength
Did you know? Arne Tiselius won the Nobel Prize in Chemistry for electrophoretic analysis of serum proteins in 1948.
Reference
• Properties of the matrix through which the molecules migrate (e.g., viscosity, pore size)
The acrylamide matrix
1. Ornstein L (1964) Disc electrophoresis. 1. Background and theory. Ann N Y Acad Sci 121:321-349.
Mini Gel Tank
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
5
6
Select precast gel
Comparison of discontinuous buffer systems SDS-PAGE utilizes a discontinuous buffer system to concentrate or “stack” samples into a very sharp zone in the stacking gel at the beginning of the run. In a discontinuous buffer system, the primary anion in the gel is different (or discontinuous) from the primary anion in the running buffer. Both the Invitrogen™ NuPAGE™ systems (Bis-Tris and Tris-acetate gels) and the Laemmli (Tris-glycine) system are examples of discontinuous buffer systems and work in a similar fashion. However, the NuPAGE system operates at a lower pH as a result of the proprietary ions that are in the system. In a Tris-glycine system (Figure 1), three ions are primarily involved: •C hloride (–), supplied by the gel buffer, serves as the leading ion because it has the highest attraction to the anode relative to other anions in the system. • Glycine (–), the primary anion provided by the running buffer, serves as the trailing ion, because it is only partially negatively charged and remains behind the more highly charged chloride ions in a charged environment. • Tris base (+), is a common ion present in both the gel and the running buffers. During electrophoresis, the gel and buffer ions in the Tris-glycine system form an operating pH of 9.5 in the separating region of the gel. In the case of the Bis-Tris system (Figure 2), three ions are primarily involved: • Chloride (–) supplied by the gel buffer, serves as the fast-moving leading ion. • MES or MOPS (–) (depending on the running buffer choice) serves as the trailing ion. ∙ MES: 2-(N-morpholino) ethane sulfonic acid ∙ MOPS: 3-(N-morpholino) propane sulfonic acid • Bis-Tris (+) acts as the common ion present in the gel while Tris (+) is provided by the running buffer.
With the Tris-acetate system (Figure 3), three ions are primarily involved: • Acetate (–), the leading ion from the gel buffer • Tricine (–), the trailing ion from the running buffer • Tris (+), the common ion (in both gel and running buffer) This system also operates at a significantly lower pH than the Trisglycine system, resulting in less gel-induced protein modifications. The diagrams below (Figures 1, 2, and 3) summarize the migration differences in the stacking gel of each system.
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Post stain
7
Select precast gel High-performance precast protein gels If you are doing standard one-dimensional protein electrophoresis, we have a broad range of solutions to fit your research needs. Our selection of precast gels consists of several different chemistries, well formats, and gel sizes, so you can get the protein separation you need for accurate downstream results. Bolt Bis-Tris Plus gel.
Learn more at thermofisher.com/proteingels GLYCINE (Trailing Ion)
PROTEIN/SDS COMPLEX (Stacked Proteins)
CHLORIDE (Leading Ion)
PROGRESSION OF RUN
Figure 1. The Tris-glycine gel system. • Gel buffer ions are Tris and chloride (pH 8.7) • Running buffer ions are Tris, glycine, and SDS (pH 8.3) • Gel operating pH is 9.5
Common Ion is Tris, present in the gel and running buffers
MES or MOPS (Trailing Ion)
PROTEIN/SDS COMPLEX (Stacked Proteins)
CHLORIDE (Leading Ion)
Figure 2. The Bis-Tris gel system. • Gel buffer ions are Bis-Tris and chloride (pH 6.4) • Running buffer ions are Tris, MES or MOPS, and SDS (pH 7.3) • Gel operating pH is 7.0
Precast gels Popular gel chemistries
Specialty gels
• NuPAGE Bis-Tris gels
• Novex Tricine gels
• NuPAGE Tris-Acetate gels
• NativePAGE gels
• Bolt Bis-Tris Plus gels
• Novex IEF gels
• Novex Tris-Glycine gels
• Novex Zymogram gels • E-PAGE gels
Did you know? Over 45 years ago, Ulrich K. Laemmli first published SDS-PAGE as a method for cleavage analysis of structural proteins in bacteriophage T4.
PROGRESSION OF RUN Common Ion is Bis-tris, present in the gel
Casting your own gels? TRICINE (Trailing Ion)
PROTEIN/SDS COMPLEX (Stacked Proteins)
ACETATE (Leading Ion)
Figure 3. The Tris-acetate gel system. • Gel buffer ions are Tris and acetate (pH 7.0) • Running buffer Ions are Tris, tricine, and SDS (pH 8.3) • Gel operating pH is 8.1
We offer preassembled empty cassettes, buffers, and reagents.
Learn more at thermofisher.com/gelcastingaccessories
PROGRESSION OF RUN Common Ion is Tris, present in the gel and running buffer
The combination of a lower-pH gel buffer (pH 6.4) and running buffer (pH 7.3–7.7) leads to a significantly lower operating pH (pH 7.0) during electrophoresis, resulting in better sample integrity and gel stability.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
8
Prepare samples and select buffers
Select precast gel
Select the standard
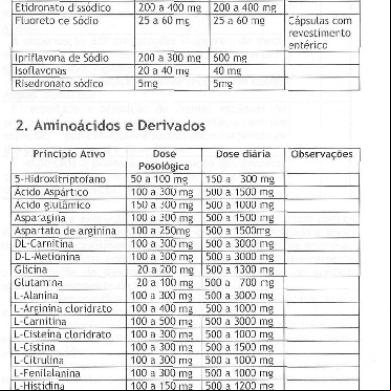
Gel selection guide
Choose the electrophoresis chamber system and power supply chamber system and power supply
Run the gel
Stain the gel
Find the right gel for your research needs based on molecular weight, downstream applications, and throughput requirements.
Molecular weight Low molecular weight proteins and peptides (>2.5 kDa)
High molecular weight proteins (<500 kDa)
Novex Tricine gels
NuPAGE Tris-Acetate gels
High-sensitivity western blotting
Broad-range molecular weight separation
Low throughput
Medium or high throughput
Application
E-PAGE 48-well or 96-well gels
Downstream applications requiring high protein integrity (e.g., mass spectrometry)
Large sample volume for high detection sensitivity
Bolt Bis-Tris Plus gels
NuPAGE Bis-Tris gels
NuPAGE Bis-Tris gels
NuPAGE Bis-Tris gels
Bolt Bis-Tris Plus gels
Bolt Bis-Tris Plus gels
Bolt Bis-Tris Plus gels
A
The most widely used gel system for separating a broad range of proteins by SDS-PAGE is the Laemmli system, which uses Tris-glycine gels comprising a stacking gel component that helps focus the proteins into sharp bands at the beginning of the electrophoretic run and the resolving gel component that separates the proteins based on size. This classic system uses a discontinuous buffer system where the pH and ionic strength of the buffer used for running the gel (Tris, pH 8.3) is different from the buffers used in the stacking gel (Tris, pH 6.8) and resolving gel (Tris, pH 8.8). The highly alkaline operating pH of the Laemmli system may cause band distortion, loss of resolution, or artifact bands. The major causes of poor band resolution with the Laemmli system are:
Novex Tris-Glycine gels
• Hydrolysis of polyacrylamide at the high pH of the resolving gel, resulting in a short shelf life of 8 weeks • Chemical alterations such as deamination and alkylation of proteins due to the high pH of the resolving gel
Native separation
Molecular weight 1st dimension 2nd dimension
Isoelectric point
NativePAGE gels
Novex Tris-Glycine gels with native buffers
Novex IEF gels
ZOOM™ IPG strips
NuPAGE Bis-Tris gels, 2D well
Novex Tris-Glycine gels, 2D well
Novex Tris-Glycine gels, 2D well
Novex Tris-Glycine ZOOM™ gels, IPG well
NuPAGE Bis-Tris gels, 2D well
NuPAGE Bis-Tris ZOOM gels, IPG well
Find the right mini gel using our interactive gel selection tool at thermofisher.com/minigelselection
For ordering information refer to pages 81–87.
Protease activity Novex Zymogram gels (casein, blue casein, or gelatin substrates)
9
Choose the right gel chemistry for your research needs Bis-Tris chemistry vs. Tris-glycine chemistry
Denaturing separation
Coomassie dye or silver staining
Protein gel electrophoresis technical handbook
Post stain
1
2
3
4
5
6
7
8
9 10
B
1
2
3
4
5
6
7
8
9
10
Figure 4. Protein separation using (A) a Bolt Bis-Tris Plus gel and (B) another manufacturer’s traditional Tris-glycine gel.
Unlike traditional Tris-glycine gels, NuPAGE and Bolt gels are BisTris HCI–buffered (pH 6.4) and have an operating pH of about 7.0. The neutral operating pH of the Bis-Tris systems provides the following advantages over the Laemmli system: • Longer shelf life of 8–12 months due to improved gel stability • Improved protein stability during electrophoresis at neutral pH enabling sharper band resolution and accurate results (Moos et al. 1998) • Complete reduction of disulfides under mild heating conditions (70°C for 10 minutes) and absence of cleavage of Asp-Pro bonds • Reduced state of the proteins maintained during electrophoresis and blotting of the proteins when using Invitrogen™ NuPAGE™ Antioxidant
• Reoxidation of reduced disulfides from cysteine-containing proteins, as the redox state of the gel is not constant • Cleavage of Asp-Pro bonds of proteins when heated at 100°C in Laemmli sample buffer, pH 5.2
Choosing the right gel percentage In general, the size of the molecule being separated should dictate the acrylamide or agarose percentage you choose. Use a lower percentage gel to resolve larger molecules and a higher percentage gel to resolve smaller ones. The exception to this rule is when performing isoelectric focusing. Refer to the gel migration charts throughout this chapter to find the gel best suited for your application. As a general rule, molecules should migrate through about 70% of the length of the gel for the best resolution. When protein molecular weights are wide ranging, or unknown, gradient gels are usually the best choice.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Choosing a well format and gel thickness We offer most polyacrylamide gels in a choice of nine different well formats (17 well, 15 well, 12 well, 10 well, 9 well, 5 well, 1 well, 2D/preparative well, or IPG well). Two thicknesses (1.0 mm and 1.5 mm) are also available for popular gel types. If loading large sample volumes (>30 μL), a thicker gel (1.5 mm) with fewer wells (e.g., 5 well) or a Bolt gel with its higher-capacity wedge wells is more appropriate. When blotting, that proteins will transfer more easily from a 1.0 mm thick gel than from a 1.5 mm thick gel.
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Bolt Bis-Tris Plus mini gels
Figure 7. Bolt Bis-Tris Plus gel migration chart. Optimal separation range is shown within the gray areas.
Neutral-pH gel system with a unique wedge well design Invitrogen™ Bolt™ Bis-Tris Plus gels are precast polyacrylamide gels designed for optimal separation of a broad molecular weight range of proteins under denaturing conditions during gel electrophoresis (Figure 6 and 7). These gels help deliver consistent performance with a neutral-pH environment to minimize protein degradation. The unique wedge well design (Figure 5) allows loading of up to 2x more sample volume than other precast gels. Bolt gels are ideal for western blot transfer and analysis along with any other technique where protein integrity is crucial. Bolt Bis-Tris Plus gels offer:
Specifications • Shelf life: ~16 months • Average run time: 35 minutes • Separation range: 0.3–260 kDa
• Better protein resolution—gels are 10% longer, allowing detection of more protein bands than standard mini gels • High lot-to-lot consistency—coefficient of variation (CV) of only 2% for Rf values (migration)
Figure 5. The unique wedge well design of Bolt Bis-Tris Plus gels.
“For one of our projects in the lab, we resolve proteins by electrophoresis to determine the accumulation of ubiquitinated proteins following treatment with a proteasome inhibitor. When we resolved the ubiquitinated proteins using the Tris-glycine gels, we observed a smear. However, when we switched to resolving the ubiquitinated proteins using the Bolt Bis-Tris gels, we were delightfully surprised to observe individual protein bands in place of the smear.” —Susan S., University of Pennsylvania, Philadelphia, US
• Polyacrylamide concentrations: fixed 8%, 10%, and 12%; gradient 4–12% • Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per 12-well gel: ~40 μL, or two-thirds of the sample well volume
• Preserved protein integrity—neutral-pH formulation minimizes protein modifications • Superior band quality and band volume— Invitrogen™ Novex™ Bis-Tris Plus chemistry is designed to deliver sharp, straight bands with higher band volume
“The new Bolt system is wonderful. I am still amazed that I can run a PAGE gel in 23 minutes. The entire system is incredibly friendly from the Bolt precast gels with wedged wells for ease of loading to the Mini Gel Tank system. The bands produced from the westerns were sharp and straight. I would and have highly recommended this system to anyone doing protein work.” — Crystal M., Queen’s University, Ontario, Canada
ts acquired w sul ith Re
• High sample volume capacity—wedge well design allows detection of proteins in very dilute samples or measurement of low-abundance proteins
11
Bolt Bis-Tris Plus gels
10
th e
Mini Gel Tan
k
Figure 6. Bolt Bis-Tris Plus gel electrophoresis. Protein standards and samples were loaded at 10 μL sample volumes in a Bolt 4–12% Bis-Tris Plus Gel. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands with consistent migration patterns were observed after staining with Invitrogen™ SimplyBlue™ SafeStain. Images were acquired using a flatbed scanner. Lane 1: Invitrogen™ SeeBlue™ Plus2 Prestained Standard; Lane 2: 10 μg E. coli lysate; Lane 3: Invitrogen™ Mark12™ Unstained Standard (blend of 12 purified proteins); Lane 4: 40 μg HeLa cell lysate; Lane 5: 20 μg HeLa cell lysate; Lane 6: 5 μg BSA; Lane 7: 40 μg Jurkat cell lysate; Lane 8: 5 μg GST fusion protein; Lane 9: Invitrogen™ Novex™ Sharp Unstained Protein Standard; Lane 10: 5 μg β-galactosidase.
Did you know? Timothy Updyke and Sheldon Engelhorn filed a patent for the neutral-pH Bis-Tris gel system in 1996.
Recommended products The Invitrogen™ Bolt™ Welcome Pack + iBlot™ 2 System offers a complete protein separation and western blot solution by combining our Mini Gel Tank, Invitrogen™ Bolt™ gels and buffers, SeeBlue Plus2 Prestained Standard, and Invitrogen™ iBlot™ 2 Gel Transfer Device with transfer stacks.
Thermo Scientific Pierce Power Stainer is recommended for fast Coomassie dye staining of Bolt Bis-Tris Plus Gels.
The Bolt Welcome Pack + iBlot 2 System.
Learn more at thermofisher.com/bolt For ordering information refer to page 81. For quick reference on the protocol please refer to page 76.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
NuPAGE gels
13
NuPAGE gels A.
Revolutionary high-performance gels referenced in >20,000 publications The Invitrogen™ NuPAGE™ SDS-PAGE gel system is a revolutionary high-performance polyacrylamide gel electrophoresis system that simulates the denaturing conditions of the traditional Laemmli system. NuPAGE™ gels use a unique buffer formulation to maintain a neutral operating pH during electrophoresis, which minimizes protein modifications that can result in poor band resolution. Gels are available in two formulations— Invitrogen™ NuPAGE™ Bis-Tris gels are ideal for separating small to midsize proteins while Invitrogen™ NuPAGE™ Tris-Acetate gels are ideal for separating large proteins (Figure 8). A gel migration chart is shown in Figure 9.
NuPAGE Bis-Tris gels (Denaturing separation)
B.
NuPAGE Tris-Acetate gels (Denaturing separation)
C.
NuPAGE Tris-Acetate gels (Native separation)
Specifications • Shelf life: –– NuPAGE Bis-Tris gels: 16 months –– NuPAGE Tris-Acetate gels: 8 months • Average run time: ~35 minutes • Separation range: –– NuPAGE Bis-Tris gels: 1.5–300 kDa –– NuPAGE Tris-Acetate gels: 30–400 kDa
NuPAGE gels are designed for:
• Polyacrylamide concentrations: –– NuPAGE Bis-Tris gels: fixed 8%, 10%, and 12%; gradient 4–12% –– NuPAGE Tris-Acetate gels: fixed 7%; gradient 3–8%
• Superior protein band resolution and stability— neutral-pH environment during electrophoresis minimizes protein modifications
• Gel dimensions: –– Mini: 8 x 8 cm (1 or 1.5 mm thick) –– Midi: 8 x 13 cm (1 mm thick)
• More efficient western blot transfer—neutral pH prevents reoxidation of reduced samples during protein transfer
• Maximum sample volume per 10-well mini gel: 25 µL (1 mm thick); 37 µL (1.5 mm thick)
• Fast sample run times—typically 35–50 minutes • Long product shelf life—stable for 8–16 months
A.
B.
ts acquired w sul ith Re
12
Figure 8. NuPAGE Bis-Tris and Tris-Acetate gel electrophoresis. Protein standards and samples were loaded at 10 μL sample volumes th e k in (A) Invitrogen™ NuPAGE™ 4–12% Bis-Tris and Mini Gel Tan (B) Invitrogen™ NuPAGE™ 3–8% Tris-Acetate gels. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands were observed after staining with SimplyBlue SafeStain. Images were acquired using a flatbed scanner. (A and B) Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 μg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 μg HeLa cell lysate; Lane 5: 20 μg HeLa cell lysate; Lane 6: (A) not used (B) 5 µg BSA; Lane 7: 40 μg Jurkat cell lysate; Lane 8: 5 μg GST fusion protein; Lane 9: Novex Sharp Unstained Protein Standard; Lane 10: 5 μg β-galactosidase.
Learn more at thermofisher.com/nupage For ordering information refer to page 81. For quick reference on the protocol please refer to page 76-77.
Figure 9. Migration patterns achieved in NuPAGE gels. For optimal results, protein bands should migrate within the gray shaded areas. (A) Migration patterns of Invitrogen™ Novex™ Sharp Prestained Protein Standard or Novex Sharp Unstained Protein Standard on NuPAGE Bis-Tris
gels. (B) Migration patterns of HiMark Unstained Protein Standard on NuPAGE Tris-Acetate gels. (C) Migration pattern for Tris-acetate gel native separation is for the Invitrogen™ NativeMark™ Unstained Protein Standard.
Recommended products Invitrogen™ HiMark™ Unstained and Prestained Protein Standards are specifically designed for large protein analysis on NuPAGE Tris-Acetate gels under denaturing conditions. Both standards offer a ready-to-load format and consist of 9 proteins with a size range of 40–500 kDa.
Visualize with Coomassie stain, silver stain, or fluorescent protein stains after electrophoresis (see “Stain the gel”, page 62).
Precast protein gels
PageRuler, PageRuler Plus, and Spectra Prestained Protein Ladders are recommended for use with NuPAGE Bis-Tris gels for easy molecular weight determination.
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Novex Tris-Glycine gels
A.
Laemmli-based precast gels for high efficiency, reproducibility, and performance
Novex Tris-Glycine gels are: • Individually packaged for convenience • Compatible with most protein standards for accurate size determination • Flexible for use with native or denatured protein samples, with specially formulated buffers for each condition
B.
Denaturing separation
Native separation††
Tris-Glycine Gels Large proteins* (116–500 kDa) 0%
Invitrogen™ Novex™ Tris-Glycine gels are based on traditional Laemmli protein electrophoresis with minor modifications for maximum performance in the precast format. These gels provide reproducible separation of a wide range of proteins into wellresolved bands (Figure 10). A gel migration chart is shown in Figure 11.
15
Novex Tris-Glycine gels
Specifications
4%
6%
Small proteins† (3–60 kDa)
Mid-size proteins† (20–250 kDa) 8%
10%
12%
14%
16%
Wide range† (6–200 kDa) 18%
4–12%
8–16%
4–20%
• Shelf life: 1–2 months
10–20%
500 kDa 260kDa kDa 260
500 kDa
10% 260 kDa
• Run time: ~90 minutes
260 kDa 290 kDa
20%
• Separation range: 6–500 kDa
260 kDa
240 kDa
30%
160 kDa
160 kDa
110 kDa
290 kDa
110 kDa 80 kDa
110 kDa
160 kDa
160 kDa 110 kDa
110 kDa
80 kDa
80 kDa
60 kDa
60 kDa
50 kDa 40 kDa
50 kDa
80 kDa 50 kDa
40%
160 kDa
30 kDa
116 kDa
15 kDa 110 kDa 10 kDa
60 kDa 30 kDa
20 kDa
th e
Mini Gel Tan
30 kDa
20 kDa 20 kDa
40 kDa
15 kDa
50 kDa 40 kDa
30 kDa
10 kDa
15 kDa
15 kDa
50 kDa
10 kDa
k
20 kDa
90%
Figure 10. Novex Tris-Glycine gel electrophoresis. Protein standards and samples were loaded at 10 μL sample volumes in 4–20% Tris-Glycine gels. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands were observed after staining with SimplyBlue SafeStain. Images were acquired using a flatbed scanner. Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 μg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 μg HeLa cell lysate; Lane 5: 20 μg HeLa cell lysate; Lane 6: 5 μg BSA; Lane 7: 40 μg Jurkat cell lysate; Lane 8: 5 μg GST fusion protein; Lane 9: Novex Sharp Unstained Protein Standard; Lane 10: 5 μg β-galactosidase.
30 kDa
50 kDa
60 kDa 10 kDa
80%
40 kDa
40 kDa
15 kDa
40 kDa
50 kDa
60 kDa
20 kDa
50 kDa
97 kDa
80 kDa
40 kDa 30 kDa
160 kDa
60 kDa 110 kDa
110 kDa
60%
50 kDa 80 kDa
20 kDa
70%
110 kDa 160 kDa
160 kDa
80 kDa
ts acquired w sul ith Re
60 kDa 260 kDa
60 kDa
• Maximum sample volume per well: 25 μL (1 mm thick); 37 μL (1.5 mm thick)
80 kDa 160 kDa
30 kDa
50 kDa
110 kDa
260 kDa
40 kDa
80 kDa 110 kDa
50%
260 kDa
40 kDa
60 kDa 240 kDa
160 kDa
260 kDa
60 kDa 160 kDa
• Gel dimensions: –– Mini: 8 x 8 cm (1 or 1.5 mm thick) –– Midi: 8 x 13 cm (1 mm thick)
260 kDa
260 kDa 160 kDa
• Polyacrylamide concentrations: –– Fixed concentrations available from 4% to 18% –– Gradient gels with ranges of 4–12%, 4–20%, 8–16%, and 10–20%
14
166 kDa
20 kDa 15 kDa
10 kDa
66 kDa
30 kDa
30 kDa 40 kDa
20 kDa
55 kDa
100%
10 kDa
15 kDa
97 kDa
15 kDa
10 kDa
Figure 11. Migration patterns of protein molecular weight standards in Novex Tris-glycine gels. For optimal results, protein bands should migrate within the gray shaded areas. (A) *Migration patterns of HiMark™ Unstained Protein Standard. † Migration patterns of Novex Sharp PreStained Protein Standard or Novex Sharp Unstained Protein Standard. (B) † † Migration pattern of NativeMARK Unstained Protein Standard.
Recommended products or sample cleanup prior to electrophoresis, we recommend using the F Pierce SDS-PAGE Sample Prep Kit.
Buffers for native proteins: Invitrogen™ Novex™ Tris-Glycine Native Sample Buffer and Novex™ Tris-Glycine Native Running Buffer.
uffers for denatured proteins: Invitrogen™ Novex™ Tris-Glycine SDS B Sample Buffer and Novex™ Tris-Glycine SDS Running Buffer.
PageRuler, PageRuler Plus, and Spectra protein ladders are recommended for molecular weight determination with Novex Tris-Glycine gels.
Learn more at thermofisher.com/trisglycine For ordering information refer to page 82–83. For quick reference on the protocol please refer to page 77-78.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
NativePAGE gels
17
NativePAGE gel
Superior resolution of native proteins and protein complexes The Invitrogen™ NativePAGE™ Bis-Tris gel system is based on the blue native polyacrylamide gel electrophoresis (BN PAGE) technique that uses Coomassie G-250 dye as a charge shift molecule that binds to proteins and confers a negative charge without denaturing the proteins (Figure 12). This technique overcomes the limitations of traditional native electrophoresis by providing a near-neutral operating pH and detergent compatibility. The near-neutral (pH 7.5) environment of the NativePAGE system during electrophoresis results in maximum protein and gel matrix stability, enabling better band resolution than other native gel systems. A gel migration chart is shown in Figure 13. The NativePAGE gel system is designed for:
Specifications • Shelf life: 6 months • Average run time: 90 minutes
Did you know?
• Separation range: 15–10,000 kDa
The blue native polyacrylamide gel electrophoresis technique was developed by Hermann Schagger and Gebhard von Jagow in 1991.
• Polyacrylamide concentrations: gradient 3–12% and 4–16% • Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per 10-well gel: 25 μL
Is there a higher res pic somewhere? I copied and pasted this from source file.
• A wide resolving range—from 15 kDa to over 10 MDa (Figure 12), regardless of isoelectric point • Neutral-pH separation—the native state of protein complexes is better preserved • Superior performance—higher resolution than Tris-glycine native electrophoresis
ts acquired w sul ith Re
dvantages of the NativePAGE gel system over A the Tris-glycine gel system include:
16
th e
Mini Gel Tan
k
Figure 13. NativePAGE gel migration chart. Migration patterns of the NativeMark Unstained Protein Standard on NativePAGE gels are shown.
Figure 12. NativePAGE gel electrophoresis. Two-fold dilution series of protein extracts were run on an Invitrogen™ NativePAGE™ Novex™ 3–12% BisTris Protein Gel using a Mini Gel Tank. Following electrophoresis, the gel was stained with Coomassie dye and imaged using a flatbed scanner. Lanes 1 and 10: blank; Lanes 2 and 6: 5 μL NativeMark Unstained Protein Standard; Lanes 3, 4 and 5: 10, 5, and 2.5 μg spinach chloroplast extract; Lanes 7, 8 and 9: 10, 5, and 2.5 μg bovine mitochondrial extract.
• Reduced vertical streaking—Coomassie G-250 dye binds to nonionic detergent molecules in the sample and carries them in the dye front, ahead of resolving proteins • Better separation of proteins—positively charged proteins with high isoelectric points are converted to proteins with a net negative charge, allowing migration to the anode
Recommended products NativeMark Unstained Protein Standard is recommended for use with native gel chemistries, including our Tris-glycine, Tris-acetate, and NativePAGE gel systems. This standard offers a wide molecular weight range of 20–1,200 kDa, and the 242 kDa β-phycoerythrin band is visible as a red band after electrophoresis for reference (prior to staining). See page 40 for details.
• Minimized protein aggregation—Coomassie G-250 dye binding allows separation of membrane proteins and proteins with exposed hydrophobic areas
Learn more at thermofisher.com/nativepage For ordering information refer to page 84. For quick reference on the protocol please refer to page 78.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Novex Tricine gels
19
Novex Tricine gel
High-resolution gels for peptide analysis and low molecular weight proteins The Invitrogen™ Novex™ Tricine gel system is a modification of the Tris-glycine system in which tricine replaces glycine in the running buffer. This system uses a discontinuous buffer system specifically designed for the resolution of low molecular weight proteins (Figure 14). Advantages of Novex Tricine gels over Tris-glycine gels include: • Increased resolution of proteins with molecular weights as low as 2 kDa (Figure 15)
Specifications • Shelf life: 1–2 months • Average run time: 90 minutes
• Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per 10-well gel: 25 μL
In the traditional Tris-glycine protein gel system, the resolution of smaller proteins (<10 kDa) is hindered by the continuous accumulation of free dodecyl sulfate (DS) ions from the SDS sample and running buffers in the stacking gel, which causes mixing of the DS ions with smaller proteins and results in fuzzy bands and decreased resolution. The mixing also interferes with the fixing and staining of smaller proteins. The Novex Tricine gel system uses a low pH in the gel buffer and sub-
Figure 15. Novex Tricine gel migration chart. For optimal resolution, protein bands should migrate within the shaded areas.
ts acquired w sul ith Re
• Minimized protein modification due to the lower pH of the tricine buffering system
How Novex Tricine gels work
Sample preparation is not the only factor that can result in poorly resolved bands. You can minimize protein degradation by using gels with neutral-pH chemistry.
• Polyacrylamide concentrations: fixed 10% and 16%; gradient 10–20%
• Improved compatibility with direct protein sequencing applications after transferring to PVDF membranes
Good to know
Did you know?
• Separation range: 2–20 kDa
18
th e
Mini Gel Tan
k
Figure 14. Novex Tricine gel electrophoresis. Protein standards and samples were loaded at 10 μL sample volumes on Invitrogen™ Novex™ 10–20% Tricine Protein Gels. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands were observed after staining with SimplyBlue SafeStain. Images were acquired using a flatbed scanner. Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 μg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 μg HeLa cell lysate; Lane 5: 20 μg HeLa cell lysate; Lane 6: 5 μg BSA; Lane 7: 40 μg Jurkat cell lysate; Lane 8: 5 μg GST fusion protein; Lane 9: Novex Sharp Unstained Protein Standard; Lane 10: 5 μg β-galactosidase.
stitutes tricine for glycine in the running buffer. The smaller proteins and peptides that migrate with the stacked DS ions
Recommended products
in the Tris-glycine gel system are well separated from DS ions
Use Novex Tricine gels with our In-Gel Tryptic Digestion Kit for separation and digestion of peptides for mass spectrometry.
in the Novex Tricine gel system, offering sharper bands and higher resolution.
Learn more at thermofisher.com/tricine For ordering information refer to page 85. For quick reference on the protocol please refer to page 79.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Novex IEF gels
21
Novex IEF gel
Precast gels for isoelectric point determination
Separated on precast vertical gel (slab) Cathode –
Isoelectric focusing (IEF) is an electrophoresis technique that separates proteins based on their isoelectric point (pI). The pI is the pH at which a protein has no net charge and does not move in an electric field. Invitrogen™ Novex™ IEF gels effectively create a pH gradient so proteins separate according to their unique pI (Figure 16 and 17). These gels can be used for pI determination or for detection of minor changes in a protein due to deamination, phosphorylation, or glycosylation, and can resolve different proteins of similar size that cannot be resolved on standard SDS-PAGE gels.
• Shelf life: 2 months
Did you know?
• Average run time: 2.5 hours
Harry Svensson-Rilbe and his student Olof Vesterberg first described the theory of separation of amphoteric proteins along a pH gradient by applying an electric field in the 1960s.
• Separation range: —pH 3–10 gels: pI performance range is 3.5–8 —pH 3–7 gels: pI performance range is 3.0–7.0 • Polyacrylamide concentration: fixed 5% • Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per 10-well gel: 20 μL
pl
1
2
3
4
5
6
7
8
9
• Higher resolution of slight differences in size when used in combination with SDS-PAGE for 2D electrophoresis
Anode +
10
8.0
• Accurate pI determination • Clear, sharp bands for easy identification of protein modifications
ts acquired w sul ith Re
When used with our convenient, pre-optimized buffers, solubilizers, and molecular weight markers, Novex IEF gels can provide:
Specifications
20
7.4
th e
Mini Gel Ta
nk
Figure 16. Novex IEF gel electrophoresis. A 2-fold dilution series 6.0 of IEF Marker 3–10 was run in duplicate on an Invitrogen™ 4.5 Novex™ pH 3–10 IEF Protein Gel using a Mini Gel Tank. The IEF Marker 3–10 consists of proteins with a variety of isoelectric points; these proteins include lectin (pI = 7.8, 8.0, and 8.3), myoglobin from horse muscle (pI = 6.9 and 7.4), carbonic anhydrase from bovine erythrocytes (pI = 6.0), β-lactoglobulin from bovine milk (pI = 5.2 and 5.3), soybean trypsin inhibitor (pI = 4.5), and glucose oxidase (pI = 4.2). After electrophoresis, the gel was fixed and stained using Coomassie R-250 dye. Gel imaging was performed with a flatbed scanner. Volume of IEF Marker 3–10 loaded: Lanes 1 and 6: 20 μL; Lanes 2 and 7: 10 μL; Lanes 3 and 8: 5 μL; Lanes 4 and 9: 2.5 μL; Lanes 5 and 10: blank.
Figure 17. Novex IEF gel migration chart using the Novex IEF marker. Proteins shown are 1: amyloglucosidase (Aspergillus niger), pI = 3.5; 2: glucose oxidase (Aspergillus niger), pI = 4.2; 3: trypsin inhibitor (soybean), pI = 4.5; 4a and 4c: β-lactoglobulin (bovine, milk), pI = 5.2 and 5.3; 5: carbonic anhydrase (bovine, erythrocytes), pI = 6.0; 6a and 6c: myoglobin (horse, muscle), pI = 6.9 and 7.4; 7a, 7m and 7c: lectin (Lens culinaris), pI = 7.8, 8.0 and 8.3; 8: ribonuclease A (bovine, pancreas), pI = 9.5; and 9: cytochrome c (horse, heart), pI = 10.7.
Recommended products Novex IEF buffer kits—includes optimized cathode, anode, and sample buffers to reduce variability and enable consistent results. IEF Marker 3–10—ready to use, enables accurate results.
ZOOM™ IEF Fractionator Combo Kit— offers a fast, reliable method to reduce sample complexity, enrich low-abundance proteins, and increase the dynamic range of detection.
Learn more at thermofisher.com/ief For ordering information refer to page 85. For quick reference on the protocol please refer to page 79.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Choose the electrophoresis chamber system and power supply
Select the standard
Novex Zymogram gels
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
23
Table 1. Novex Zymogram gels available. Novex Zymogram gel Novex Zymogram gelatin gel
Easy in-gel protease analysis Invitrogen™ Novex™ Zymogram gels are excellent tools for detecting and characterizing proteases that utilize casein or gelatin as a substrate. Casein and gelatin are the most commonly used substrates for demonstrating the activity of proteases. Novex Zymogram gels are used to analyze a variety of enzymes, including matrix metalloproteinases, lipases, and other proteases (Figure 18). Available gel types are shown in Table 1.
Novex Zymogram casein gel
Novex Zymogram blue casein gel
Gel composition
10% TrisGlycine gel
12% TrisGlycine gel
4–16% TrisGlycine gel
Substrate
0.1% gelatin
0.05% casein
0.1% casein, with blue stain incorporated in gel
Protease analysis 10% gel (w/gelatin)
12% gel (w/casein)
4—16% gel (w/prestained casein blue)
10
20
Sensitivity
10–6 units of collagenase
7 x 10 –4 units of trypsin
1.5 x 10 –3 units of trypsin
How do Novex Zymogram gels work?
Post-staining required?
Yes
Yes
No
Protease samples are denatured in SDS buffer under nonre-
Separation range
20–120 kDa
30
Good to know
1
40
30–150 kDa
10–220 kDa
Zymogram gel using Novex Tris-Glycine SDS Running Buffer. After electrophoresis, the enzyme is renatured by incubating the gel in Invitrogen™ Novex™ Zymogram™ Renaturing Buffer
Specifications
that contains a nonionic detergent. The gels are then equili-
• Shelf life: 2 months
brated in Invitrogen™ Novex™ Zymogram™ Developing Buffer to add divalent metal cations required for enzymatic activity, and then stained and destained. Regions of protease activity appear as clear bands against a dark blue background where the protease has digested the substrate.
% of length of gel
ducing conditions and without heating, and run on a Novex
50 2 1
60
2
2
3 3
• Average run time: 90 minutes • Separation range: 10–220 kDa (Figure 19) • Polyacrylamide concentrations: fixed 10% (with gelatin), fixed 12% (with casein); gradient 4–16% (with blue casein)
70
Figure 19. Novex Zymogram gel migration chart. The numbered bands refer to the following proteases: Band 1: Collagenase Type I (140 kDa) Band 2: Thermolysin (37 kDa) Band 3: Chymotrypsin (30 kDa) Band 4: Trypsin (19 kDa)
4
3
80
4
2
• Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per well: 20 μL
2
3
4
5
6
7
8
9 10
4
90
ts acquired w sul ith Re
1
22
Figure 18. Novex Zymogram gel electrophoresis. Type I collagenase was run in duplicate on an Invitrogen™ Novex™ 10% Zymogram (Gelatin) Protein th e k Gel using a Mini Gel Tank. The gel was developed Mini Gel Tan using Novex Zymogram Renaturing Buffer and Novex Zymogram Developing Buffer and stained using SimplyBlue SafeStain. Images were acquired using a flatbed scanner. Lanes 3 and 7: 5 μL of 2.0 μU/mL type I collagenase; Lanes 1, 4, 5, and 10: 12 μL SeeBlue Prestained Protein Standard.
4
100
Recommended products After electrophoresis, incubate the gel in Zymogram Renaturing Buffer to renature the enzyme. The gels are then equilibrated in Zymogram Developing Buffer to add divalent metal cations required for enzymatic activity.
Learn more at thermofisher.com/zymogram For ordering information refer to page 85. For quick reference on the protocol please refer to page 80.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
24
Prepare samples and select buffers
Select precast gel
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
E-PAGE High-Throughput Precast Gel System
E-PAGE gel
E-PAGE 48 8% Gel
Protein separation and analysis for increased sample throughput
E-PAGE 96 6% Gel
0%
0% 220 kDa
10%
The Invitrogen E-PAGE High-Throughput Precast Gel System is designed for fast, bufferless medium- and high-throughput protein analysis. Invitrogen™ E-PAGE™ 48-well and 96-well precast gels consist of a buffered gel matrix and electrodes packaged inside a disposable, UV-transparent cassette. Each cassette is labeled with a unique barcode to facilitate identification of the gel using commercial barcode readers. These gels can be loaded by multichannel pipettor or automated loading system. The E-PAGE system also includes E-Base™ integrated devices to run the gels, an E-Holder™ platform for optional robotic loading, and free E-Editor™ 2.0 Software to align images for easy comparison. ™
220 kDa
™
Advantages of using the E-PAGE High-Throughput Precast Gel System include: • Ease-of-use—quick setup and fast protein separation in about 23 minutes
25
120 kDa
25%
Did you know?
20% 120 kDa 30%
100 kDa
60 kDa 40 kDa
80 kDa
75% 20 kDa
40%
Specifications
50%
60 kDa
• Shelf life: 6 months
50%
• Average run time: 14 minutes
50 kDa
100%
Figure 21. E-PAGE gel migration chart. Migration patterns of the Invitrogen™ E-PAGE™ MagicMark™ Unstained Protein Standard are shown.
Our E-Base devices are compatible with the Society for Biomolecules Screening (SBS) standard plate size and can be conveniently mounted on liquid handling robot decks.
40 kDa 60%
• Separation range: 10–200 kDa
30 kDa
• Polyacrylamide concentrations: –– E-PAGE™ 48 gel: fixed 8%
70% 20 kDa
–– E-PAGE™ 96 gel: fixed 6%
80%
• Gel dimensions: 13.5 x 10.8 cm (3.7 mm thick) • Maximum sample volume per well: –– E-PAGE 48 gel: 20 µL –– E-PAGE 96 gel: 15 µL
90%
100%
B
A
• Fast loading—compatible with multichannel pipettors and robotic loading
Recommended products
• Efficient western blotting and staining—optimized protocols and reagents
The E-PAGE™ SeeBlue™ Prestained Protein Standard or E-PAGE MagicMark Unstained Protein Standard are specifically designed for use with E-PAGE gels.
Good to know
C Mother E-Base
E-PAGE 96 gels
How do E-PAGE gels work? E-PAGE gels run in the Invitrogen™ E-Base electrophoresis de-
Daughter E-Base
vice, which has an integrated power supply for direct connection to an electrical outlet. Use the Invitrogen™ Mother E-Base™ device for a single E-PAGE gel, or use the Mother E-Base device in conjunction with two or more Invitrogen™ Daughter
Figure 20. Loading and running E-PAGE gels. (A) Loading E-PAGE 48 gels using a multi-channel pipettor. (B) Loading E-PAGE 96 gels using a multi-channel pipettor. (C) The Mother/Daughter E-Base combination.
E-Base™ devices for running multiple gels simultaneously.
Learn more at thermofisher.com/epage For ordering information refer to page 85.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Prepare the sample Sample prep kits
High salt concentration in samples: High salt concentrations result in increased conductivity that affects protein migration, and
Before a sample can be loaded onto a gel for analysis, it must be properly prepared. Depending on the gel type, this may involve denaturing the proteins, reducing any disulfide bonds, adjusting the ionic strength, and removing interfering contaminants. General guidelines for preparing samples are provided below. General guidelines for preparing samples: Prepare your sample in the appropriate sample buffer such that the final concentration of the sample buffer is 1X. Recommended sample buffers are listed on page 29. Running reduced and non-reduced samples: For optimal
Protein gel electrophoresis technical handbook
Post stain
can result in gel artifacts in adjacent lanes containing samples with normal salt concentrations. Perform dialysis or precipitate and resuspend samples in lower-salt buffer prior to electrophoresis.
Pierce SDS-PAGE Sample Prep Kit
Sample treated with the Pierce SDS-PAGE Sample Prep Kit
Untreated M
S
M
S
M
S
M
Guanidine-HCl in samples: Samples solubilized in guanidineHCl have high ionic strength, and produce increased conductivity similar to high salt concentrations. In addition, guanidine precipitates in the presence of SDS leading to various types of gel artifacts. If possible, change the solubilization agent by dialysis prior to electrophoresis.
Cell lysates Consider the following when performing electrophoresis of cell lysates: • Genomic DNA in the cell lysate may cause the sample to become viscous and affect protein migration patterns and resolution. Shear genomic DNA to reduce viscosity before loading the sample. • Cells lysates contain soluble and insoluble fractions. The size of each fraction depends on the type of sample being analyzed. The nature of the insoluble fraction may result in altered protein migration patterns and resolution. Separate the two fractions by centrifugation and load them on separate lanes for electrophoresis. • If radioimmunoprecipitation assay (RIPA) buffer is used in cell lysis, subsequent blotting of proteins less than 40 kDa may be inhibited due to the presence of Triton™ X-100 in the buffer.
A protein sample can be purged of any contaminants typically in only 10 minutes using the Thermo Scientific™ Pierce™ SDS-PAGE Sample Prep Kit. This is much faster than dialysis or ultrafiltration and yields higher protein recoveries while concentrating the sample. Advantages of using the Pierce SDS-PAGE Sample Prep Kit include: • Eliminates artifacts caused by incompatible contaminants—removes dyes, reducing agents, detergents, sugars, glycerol, guanidine, urea, and ammonium sulfate to provide reproducible results for SDS-PAGE analysis (Figure 22) • Compatible with the Thermo Scientific™ Pierce™ BCA Assay—allows quantification of the processed sample • Enriches dilute protein solutions—concentrates protein sample by eight-fold in less than 20 minutes for SDS-PAGE analysis (Figure 22) • Fast and easy to use for up to 70 μg of protein per sample—uses new spin cup format that allows higher amounts of protein to be processed than with the original procedure
Figure 22. Minimize distortion caused by detergents. Rat C6 cells were lysed and a membrane protein fraction isolated using the Thermo Scientific™ MemPER™ Eukaryotic Membrane Protein Extraction Reagent. Membrane and hydrophilic cell fractions were separated by SDS-PAGE using 4–20% gradient gels with or without prior treatment using the Pierce SDS-PAGE Sample Prep Kit. Western blot analysis was performed using an antibody against cytochrome oxidase subunit 4 (COX4) and Thermo Scientific™ SuperSignal™ West Femto chemiluminescent substrate. Kit-treated samples exhibit better band straightness and resolution with low molecular weight proteins than samples that were untreated. S = Soluble fraction (hydrophilic) M = Membrane fraction 100
Figure 23. Consistent 88% 80 protein 85% 77% 77% recovery is 75% 74% achieved 60 using the Pierce SDS40 PAGE Sample Prep Kit. 20 Pure proteins (60 µg) of 0 assorted Carbonic Ovalbumin Transferrin Ubiquitin Cytochrome c Bacterial anhydrase lysat e molecular mass (30, 44, 80, 86, and 120 kDa) as well as a bacterial lysate were processed using this kit. Protein concentrations were determined with the Pierce BCA Protein Assay Kit and reported as percent protein recovered. Table 2. Interfering substances effectively removed.
Good does to know How it work?
For quick protein clean-up and enrichment for SDS-PAGE we
samples on the same gel. If you do choose to run reduced and
recommend using the Thermo Scientific Pierce SDS-PAGE Sample
non-reduced samples on the same gel, do not run reduced and
Prep Kit, which removes substances such as guanidine-HCL
Our Pierce SDS-PAGE Sample Prep Kit uses a unique resin
non-reduced samples in adjacent lanes. The reducing agent may
and ionic detergents that can result in protein bands that appear
of modified diatomaceous earth that binds protein in DMSO.
have a carry-over effect on the non-reduced samples if they are in
smeared or wavy in the gel or on a western blot.
Simply combine 2–300 μL of sample containing up to 70 μg of
Interfering reagents
Percent protein recovered (Starting amount = 20 µg BSA)
Control (water)
75%
0.5 M Sodium chloride
80%
2 M Ammonium sulfate
76%
protein with 20 μL of Pierce™ SDS Protein Binding Resin and
20% SDS
75%
DMSO. After the proteins bind to the resin, wash away the
10% Triton™ detergent
75%
unbound contaminating chemicals. Finally, elute the sample
6 M Urea:DMSO (1:3 ratio)
75%
buffer results in proteolysis (Kubo, 1995). We recommend heating
in 50 μL of the Elution Buffer. The recovered protein sample is
1 M Sodium chloride
75%
samples for denaturing electrophoresis (reduced or non-reduced)
ready to mix with the supplied Sample Loading Buffer for
6 M Urea
74%
at 85°C for 2–5 minutes for optimal results. Do not heat the
gel loading.
10% CHAPS
80%
25% Glycerol
71%
10% OTG
71%
2 M Guanidinium•HCl
70%
40% Sucrose
70%
Heating samples: Heating the sample at 100°C in SDS-containing
samples for non-denaturing (native) electrophoresis or Novex Zymogram Gels.
Learn more at thermofisher.com/PAGEsampleprep
For ordering information refer to page 85.
S
Quick protein clean-up and enrichment for SDS-PAGE
results, we do not recommend running reduced and non-reduced
close proximity.
27
Percent protein recovered
26
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
28
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Select buffers
29
Recommended SDS-PAGE buffers and reagents
Buffers and reagents
Protein samples prepared for PAGE analysis are denatured by heating in the presence of a sample buffer with or without a reducing agent. The protein sample is mixed with the sample buffer and heated for 2–10 minutes, then cooled to room temperature before it is applied to the sample well on the gel. Loading buffers also contain glycerol so that they are heavier than water and sink neatly to the bottom of the buffer-submerged well when added to a gel.
Protein gel electrophoresis technical handbook
Post stain
Gel type
If suitable, negatively charged, low molecular weight dye is also included in the sample buffer; it will migrate at the buffer front, enabling one to monitor the progress of electrophoresis. The most common tracking dye for sample loading buffers is bromophenol blue. We offer premixed, reliable SDS-PAGE buffers and reagents including sample buffers, running buffers, reducing agents, and antioxidants.
Learn more at
Bolt Bis-Tris Plus Gel
Sample buffer optimized for use with the gel
Other compatible sample buffers
Running buffer optimized for use with the gel
• Bolt™ Sample Reducing Agent (10X) • Bolt™ LDS Sample Buffer (4X) (nonreducing) • Bolt Antioxidant
• Pierce™ LDS Sample Buffer (4X) for storage at RT
• Bolt™ MES SDS Running Buffer (20X) • Bolt™ MOPS SDS Running Buffer (20X)
NuPAGE Bis-Tris Gel
• NuPAGE Sample Reducing Agent (10X) • NuPAGE Antioxidant • NuPAGE™ LDS Sample Buffer (4X) (nonreducing)
NuPAGE Tris-Acetate Gel
• Novex Tris-Glycine SDS Sample Buffer (2X) • NuPAGE Sample Reducing Agent (10X) • Novex Tris-Glycine Native Sample Buffer (2X)
Novex Tris-Glycine Gel
• Novex Tris-Glycine SDS Sample Buffer (2X) • NuPAGE Sample Reducing Agent • Novex Tris-Glycine Native Sample Buffer (2X)
™
• Pierce™ Lane Marker Non• NuPAGE™ MES SDS Reducing Sample Running Buffer (20X) Buffer (5X)— • NuPAGE™ MOPS SDS storage at RT; Running Buffer (20X) when you desire to dilute your sample less and require transferable marker dye to nitrocellulose membranes • Pierce™ Lane Marker Reducing Sample Buffer (5X)—when you desire to dilute your sample less and require transferable marker dye to nitrocellulose membranes
MES vs. MOPS Running Buffer: • Use MES SDS running buffers to resolve small molecular weight proteins. • Use MOPS running buffers to resolve mid-size proteins. MES has a lower pKa than MOPS, enabling gels with MES running buffer to run faster than gels with MOPS SDS running buffer. The difference in ion migration affects stacking and results in a difference in protein separation range between these buffers.
• NuPAGE™ Tris-Acetate SDS Running Buffer (20X) • Novex Tris-Glycine Native Running Buffer (10X)
Reducing agent: When preparing samples for reducing gel electrophoresis, any of the following reducing agents may be used: •B olt Sample Reducing Agent
• Novex Tris-Glycine SDS Running Buffer (10X) • Novex Tris-Glycine Native Running Buffer (10X) • Pierce™ Tris-Glycine SDS Buffer (10X) • BupH™ Tris-Glycine-SDS Buffer Packs
•N uPAGE Sample Reducing Agent
Novex Tricine Gel
• Novex™ Tricine SDS Sample Buffer (2X)
• Novex Tricine SDS Running Buffer (10X)
NativePAGE Gel
• NativePAGE™ Sample Buffer (4X) • NativePAGE™ 5% G-250 Sample Additive
• NativePAGE™ Running Buffer (20X) • NativePAGE™ Cathode Buffer Additive (20X)
Novex IEF Gel
• Novex™ IEF Sample Buffer, pH 3–10 (2X) • Novex™ IEF Sample Buffer, pH 3–7 (2X)
• Novex™ IEF Anode Buffer (50X) • Novex™ IEF Cathode Buffer, pH 3–10 (10X) • Novex™ IEF Cathode Buffer, pH 3–7 (10X)
Novex Zymogram Gels*
• Novex Tris-Glycine SDS Sample Buffer (2X)
• Novex Tris-Glycine SDS Running Buffer (10X)
ithiothreitol (DTT), 50 mM final D concentration • β-mercaptoethanol (β-ME), 2.5% final concentration • tris(2-carboxyethyl)phosphine (TCEP), 50 mM final concentration Add the reducing agent to the sample up to an hour before loading the gel. Avoid storing reduced samples for long periods, even if they are frozen. Reoxidation of samples can occur during storage and produce inconsistent results.
*Novex Zymogram Developing Buffer (10X) and Novex Zymogram Renaturing Buffer (10X) are available for visualizing the Novex Zymogram gels.
thermofisher.com/electrophoresisbuffers For ordering information refer to page 85.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
30
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
31
Buffer recipes NuPAGE buffer recipes
Tris-glycine buffer recipes
Buffer
Storage
NuPAGE LDS Sample Buffer
+4˚–25˚C
NuPAGE MOPS SDS Running Buffer*
Component
Concentration (1X)
Glycerol Tris base Tris HCl LDS EDTA SERVA™ Blue G-250 Phenol Red
0% 141 mM 106 mM 2% 0.51 mM 0.22 mM 0.175 mM (pH 8.5)
NuPAGE Tris-Acetate SDS Running Buffer
Tris-Glycine SDS Sample Buffer
+4˚C
Tris-Glycine Native Sample Buffer
50 mM 50 mM 0.1% 1 mM (pH 7.7)
Tris-Glycine SDS Running Buffer
50 mM 50 mM 0.1% 1 mM (pH 7.3)
Tris-Glycine Native Running Buffer
Room temperature
Room temperature
+4˚–25˚C Bicine Bis-Tris (free base) EDTA Chlorobutanol
25 mM 25 mM 1.0 mM 0.05 mM (pH 7.2)
Tris base Tricine SDS
50 mM 50 mM 0.1% (pH 8.24)
Tris-Glycine Transfer Buffer
Component
Concentration (1X)
Tris HCl* Glycerol SDS Bromophenol Blue Deionized water
63 mM 10% 2% 0.0025% — (pH 6.8)
Tris HCl* Glycerol Bromophenol Blue Deionized water
100 mM 10% 0.0025% — (pH 8.6)
Tris base Glycine SDS Deionized water
25 mM 192 mM 0.1% — (pH 8.3)
Tris base Glycine Deionized water
25 mM 192 mM — (pH 8.3)
Tris base Glycine Deionized water
12 mM 96 mM — (pH 8.3)
+4˚C
+4˚–25˚C MES Tris base SDS EDTA
NuPAGE™ Transfer Buffer
Storage
+4˚–25˚C MOPS Tris base SDS EDTA
NuPAGE MES SDS Running Buffer*
Buffer
Room temperature
+4˚–25˚C * Tris HCl solutions are prepared from Tris base and pH adjusted with 6 N HCl.
* The pre-mixed buffers (Cat. Nos. NP0001 and NP0002) also contain trace amounts of the proprietary NuPAGE Antioxidant (Cat. No. NP0005) for stability. Additional Antioxidant may be required with specific protocols.
For ordering information refer to page 85.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
32
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
33
Buffer recipes Isoelectric focusing buffer recipes
Tricine buffer recipes Buffer
Storage
Tricine SDS Sample Buffer
+4˚C
Tricine SDS Running Buffer
Room temperature
Component
Concentration (1X)
Tris HCl* Glycerol SDS Coomassie Blue G Phenol Red Deionized water
450 mM 12% 4% 0.0075% 0.0025% – (pH 8.45)
Tris base Tricine SDS Deionized water
100 mM 100 mM 0.1% – (pH 8.3)
Buffer
Storage
IEF Sample Buffer pH 3-7
+4˚C
IEF Sample Buffer pH 3-10
+4˚C
* Tris HCl solutions are prepared from Tris base and pH adjusted with 6 N HCl.
IEF Cathode Buffer pH 3-10 (upper buffer chamber)
+4˚C
Zymogram buffer recipes
IEF Anode Buffer (for both pH ranges) (lower buffer chamber)
Room temperature
Urea-Thiourea-CHAPS (rehydration buffer for IPG strips)
–20˚C
Storage
Zymogram Renaturing Buffer
Room temperature
Zymogram Developing Buffer
Room temperature
* Tris HCl solutions are prepared from Tris base and pH adjusted with 6 N HCl.
For ordering information refer to page 85.
Component
Concentration (1X)
Triton™ X-100 Deionized water
2.7% (w/v) in H2O
Tris HCI* NaCl CaCl2•2 H2O Brij™ 35 Deionized water
50 mM 200 mM 5 mM 0.006% (w/v) _ (pH 7.6)
Concentration (1X)
Lysine (free base)
40 mM
Glycerol Deionized water
15% —
Arginine (free base) Lysine (free base) Glycerol Deionized water
20 mM 20 mM 15% —
Lysine (free base) Deionized water
40 mM —
Arginine (free base) Lysine (free base) Deionized water
20 mM 20 mM — (pH 10.1)
Phosphoric acid 85% Deionized water
7 mM — (pH 2.4)
Deionized urea Deionized thiourea CHAPS Ampholytes* Bromophenol Blue
7M 2M 2–4% 0.2–2.0% 0.002%
Ultrapure water
—
DTT
20 mM
+4˚C
IEF Cathode Buffer pH 3-7 (upper buffer chamber)
Buffer
Component
* For ZOOM™ Strip pH 9-12 use 1% ZOOM™ Focusing Buffer pH 7-12 instead of ampholytes.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
34
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
35
Select the standard Protein ladders and standards To assess the relative molecular weights (sizes) of proteins in a sample, a mixture containing several proteins of known molecular mass are run alongside the test sample lane(s). Often these protein mixtures are run on the outer lanes of the gel, to maximize the number of remaining gel wells for test samples, but can also be useful in the middle wells of the gel when running a large gel with many wells. Such sets of known protein mixtures are called protein molecular weight markers or protein ladders. It is important to choose a protein ladder that consists of proteins with molecular weights that span the molecular weight range of the protein(s) of interest. A standard curve can be constructed from the distances
each marker protein migrates through the gel. After measuring the migration distance that an unknown protein travels through the same gel, its molecular weight can be determined graphically from the standard curve. Several kinds of ready-to-use protein molecular weight (MW) markers are available that are labeled, prestained, or unstained for different modes of detection and downstream applications. We offer ladders suitable for both SDSPAGE as well as native PAGE.
Unstained protein ladders
Ready-to-use prestained and unstained protein ladders with exceptional lot-to-lot consistency
Low range
PageRuler Unstained Low Range Protein Ladder
Broad range
PageRuler Unstained Protein Ladder
We offer a broad range of prestained and unstained protein
High range
NativeMark Unstained Protein Standard
ladders supplied in a ready-to-use format to facilitate easy protein
Recommended for: • Precise determination of target protein molecular weight
analysis during gel electrophoresis and western blotting (Table 3). All of our protein ladders offer: • Performance—sharp protein bands and consistent migration patterns provide easy molecular weight determination
Prestained protein ladders Low range
PageRuler Prestained Protein Ladder
• Convenience—protein ladders are ready to load, with no heating required
Broad range
PageRuler Plus Prestained Protein Ladder Spectra™ Multicolor Broad Range Protein Ladder
• Reliability—exceptional lot-to-lot consistency and reproducibility
High range
HiMark Prestained Protein Standard Spectra Multicolor High Range Protein Ladder
Recommended for: • Approximate determination of molecular weight • Monitoring the progress of electrophoresis runs • Estimating the efficiency of protein transfer to the membrane during western blotting
Learn more at thermofisher.com/proteinstandards
Other Western
MagicMark XP Western Protein Standard
Specialty
PageRuler Prestained NIR Protein Ladder BenchMark Fluorescent Protein Standard BenchMark His-tagged Protein Standard IEF Marker 3-10
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
36
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
37
Table 3. Protein standard selection guide Category
Range
No. of bands
Reference bands
Protein MW determination
Protein band visualization
Monitoring electrophoresis run
Coomassie dye, silver, Monitoring protein or fluorescent staining transfer
Chemiluminescent band visualization
PageRuler Unstained Low Range Protein Ladder
3.4–100 kDa
8
25 kDa
Best
NA
NA
Best
NA
Good
PageRuler Unstained Protein Ladder
10–200 kDa
14
50 kDa
Good
NA
NA
Good
NA
Good
NativeMark Unstained Protein Standard
20–1,200 kDa
8
Best for native electrophoresis
NA
NA
Best
NA
Good
PageRuler Prestained Protein Ladder
10–180 kDa
10
Green 10 kDa; orange 70 kDa
Good
Good
Good
NA
Good
Good
PageRuler Plus Prestained Protein Ladder
10–250 kDa
9
Green 10 kDa; orange 25 and 70 kDa
Good
Good
Good
NA
Good
NA
HiMark Prestained Protein Standard
30–460 kDa
9
Best for high MW proteins
Good
Good
NA
Best for high MW proteins
NA
Product
Unstained ladders and standards Unstained standards
Pretained protein ladders Prestained protein standards
Spectra Multicolor Broad Range 10–260 kDa Protein Ladder
10
Green 10 and 50 kDa; orange 40, 70, and 260 kDa; pink 140 kDa
Good
Best
Best
NA
Best
NA
Spectra Multicolor High Range Protein Ladder
40–300 kDa
8
Green 50 kDa; orange 70 and 300 kDa
Good
Best
Best
NA
Best
NA
Other ladders and standards IEF
IEF Marker 3-10
pI 3.5–10.7
13
Best for pI estimation
NA
NA
Good
NA
NA
Chemiluminescent standard
MagicMark XP Western Protein Standard
20–220 kDa
9
Good
NA
NA
Good
NA
Best
Near infrared (NIR) standard
PageRuler Prestained NIR Protein Ladder
11–250 kDa
10
Good
NA
NA
NA
NA
NA
Fluorescent standard
BenchMark Fluorescent Protein Standard
11–155 kDa
7
Good
NA
NA
NA
NA
Good
His-tag standard
BenchMark His-tagged Protein Standard
10–160 kDa
10
Best
NA
NA
Good
NA
Good for detection with anti-His antibody
55 kDa
Learn more at thermofisher.com/proteinstandards
For ordering information refer to page 86.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
38
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
39
Unstained ladders and standards
PageRuler Unstained Low Range Protein Ladder
PageRuler Unstained Protein Ladder
Sharp bands and precise molecular weight estimation for low molecular weight proteins
Sharp bands and precise molecular weight estimation for a wide range of proteins
Thermo Scientific™ PageRuler™ Unstained Low Range Protein Ladder is a mixture of eight proteins and peptides for use as size standards that resolve into clearly identifiable sharp bands when analyzed by SDS-PAGE. The proteins (except for the 5 and 3.4 kDa peptides) contain an integral Strep-tag™ II Sequence and may be detected on western blots using Strep-Tactin™ Conjugates. • Comprehensive—eight proteins and peptides spanning 3.4 to 100 kDa; the 25 kDa band is more intense than the other bands for easy orientation • Versatile—compatible with western blots by staining with Ponceau S dye or Coomassie dye; compatible with Thermo Scientific™ Pierce™ Reversible Protein Stain Kit for Nitrocellulose Membranes or other protein stains
PageRuler Unstained Low Range Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The PageRuler Unstained Protein Ladder is recommended for Novex Tris-Glycine, Bis-Tris or Tris-Acetate gels.
Thermo Scientific™ PageRuler™ Unstained Protein Ladder is a mixture of 14 recombinant, highly purified, unstained proteins for use as size standards in SDS-PAGE and western blotting. Each protein in the ladder contains an integral Strep-tag II Sequence, which can be detected directly on western blots using a Strep-Tactin Conjugate or an antibody against the Strep-tag II Sequence. • Comprehensive—14 highly purified proteins with excellent accuracy spanning 10 to 200 kDa; the ladder contains one 50 kDa reference band of higher intensity • Versatile—compatible with Coomassie dye; compatible with Pierce Reversible Protein Stain Kit for Nitrocellulose Membranes, silver staining, or western blotting
Learn more at
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The PageRuler Unstained Protein Ladder is recommended for Novex Tris-Glycine, Bis-Tris or Tris-Acetate gels.
Learn more at
thermofisher.com/unstainedstandards
For ordering information refer to page 86.
PageRuler Unstained Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
thermofisher.com/unstainedstandards
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
40
Prepare samples and select buffers
Select precast gel
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
41
Prestained ladders
NativeMark Unstained Protein Standard Convenient molecular weight estimation for native electrophoresis
kDa kDa
1,0481,048 1,2361,236 1,0481,048
720 720 480 480
720 720 242 242 480 480 146 146 242 242
The Invitrogen NativeMark Unstained Protein Standard is designed for molecular weight estimation of proteins using native gel electrophoresis. ™
™
• Comprehensive—contains a wide range of high molecular weight proteins, providing 8 protein bands in the range of 20–1,200 kDa • Versatile—can be visualized using Coomassie, silver, or fluorescent stains after electrophoresis, or with Ponceau S, Coomassie, or other membrane stains after western transfer
PageRuler Prestained Protein Ladder
kDa kDa 1,2361,236
66 66
146 146
NativeMark Unstained Protein Standard NativePAGE Bis-Tris gels
66 66 20 20 20 20
3–12% 3–12%
4–16% 4–16%
Storage specifications • Storage buffer: Bis/Tris-HCl (pH 7.0), NaCl, glycerol, and Ponceau S • Storage conditions: upon receipt store at –20°C • Stability: 6 months
Recommended products The NativeMark Unstained Protein Standard is recommended for use with NativePAGE Bis-Tris gels, Novex Tris-Glycine gels, or NuPAGE Tris-Acetate gels.
Outstanding clarity for easy molecular weight determination of low molecular weight proteins Thermo Scientific™ PageRuler™ Prestained Protein Ladder is a mixture of 10 blue-, orange-, and green-stained proteins for use as size standards in SDS-PAGE and western blotting. The mobility of prestained proteins can vary in different SDSPAGE buffer systems; however, they are suitable for approximate molecular weight determination when calibrated against unstained standards in the same system. • Comprehensive—contains 10 proteins with a range of 10 to 180 kDa; includes one 70 kDa reference protein colored with an orange dye and one 10 kDa reference protein colored with a green dye
PageRuler Prestained Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The PageRuler Prestained Protein Ladder is recommended for use with Tris-glycine, Bis-Tris, and Tris-acetate gels.
• Versatile—compatible with Coomassie dye staining and western blotting
Learn more at
Learn more at
thermofisher.com/prestainedstandards
thermofisher.com/unstainedstandards
For ordering information refer to page 86.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
42
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
PageRuler Plus Prestained Protein Ladder Outstanding clarity for easy molecular weight determination of a broad range of proteins
Protein gel electrophoresis technical handbook
Post stain
HiMark Prestained Protein Standard PageRuler Plus Prestained Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
• Comprehensive—9 proteins with a broad range of 10 to 250 kDa; includes 70 kDa and 25 kDa reference proteins that are colored with an orange dye and one 10 kDa reference protein that is colored with a green dye
kDa
198
220
98
268 238
62
171
117
• Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C
110 80
60
50
60 40 30
40
28
20
71
17
15 HiMark Prestained Protein Standard NuPAGE 3–8%10Tris-acetate SDS buffer 30
14
Standard
160
80
50
38
™
260
120 100
49
The Invitrogen HiMark Prestained Protein Standard is designed for analysis of high molecular6 3 weight proteins on NuPAGE Tris-acetate gels.
Storage specifications
kDa
kDa
460
Superb analysis of high molecular weight proteins ™
Thermo Scientific™ PageRuler™ Plus Prestained Protein Ladder is a mixture of 9 blue-, orange-, and green-stained proteins for use as size standards in SDS-PAGE and western blotting. The mobility of prestained proteins can vary in different SDS-PAGE buffer systems; however, they are suitable for approximate molecular weight determination when calibrated against unstained standards in the same system.
kDa
43
55
20
41
3.5
31
SeeBlue® Plus2
HiMark™ Pre-stained
MagicMark™ XP
Novex® Sharp Pre-stained
• Comprehensive—contains a wide range of high molecular Cat. No. LC5800 LC5602 LC5925 LC5699 weight proteins, providing 9 protein bands in the range of NuPAGE 3–8% NuPAGE NuPAGE NuPAGE Bis-Tris Gel, Storage Tris-Acetatespecifications Gel blotted to 4–12% Bis-Tris 4–12% Bis-Tris w/ Tris-acetate nitrocellulose, Gel w/ MES Gel w/ MES 30–460 kDa SDS buffer detected w/ SDS buffer SDS buffer WesternBreeze • Storage buffer: Tris-HCl, formamide, SDS, and phenol red Chemiluminescent Kit • Versatile—easy visualization of band migration during • Storage conditions: upon receipt store at –20°C electrophoresis and rapid evaluation of western transfer efficiency • Stability: 6 months from date of receipt ®
®
®
®
®
• Stability: 1 year from date of receipt
Recommended products Recommended products
The HiMark Prestained Protein Standard is recommended for use with NuPAGE Tris-Acetate gels under denaturing conditions. This standard can also be used with NuPAGE 4–12% Bis-Tris gels with Invitrogen™ NuPAGE MOPS SDS Running Buffer and Novex 4% Tris-Glycine gels. However, to obtain the best results with high molecular weight proteins, always use NuPAGE Tris-Acetate gels.
The PageRuler Plus Prestained Protein Ladder is recommended for Trisglycine, Bis-Tris, and Tris-acetate gels.
• Versatile—compatible with Coomassie dye staining and western blotting
Learn more at
The HiMark Prestained Protein Standard is also available as part of the following kits that include gels, running and sample buffers, and stains or blotting materials: • Invitrogen™ NuPAGE™ Large Protein Staining Kit • Invitrogen™ NuPAGE™ Large Protein Sensitive Staining Kit • Invitrogen™ NuPAGE™ Large Protein Blotting Kit
thermofisher.com/prestainedstandards
Learn more at thermofisher.com/prestainedstandards
For ordering information refer to page 86.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
44
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Spectra Multicolor Broad Range Protein Ladder
Spectra Multicolor High Range Protein Ladder
Superior visualization and analysis of a broad range of proteins
Superior and convenient visualization of high molecular weight proteins
Thermo Scientific Spectra Multicolor Broad Range Protein Ladder is a 4-color protein standard containing 10 prestained proteins for use in gel electrophoresis and western blotting. This standard is designed for monitoring the progress of gels during SDS-PAGE and for assessing western blot transfer efficiency. Four different chromophores (blue, orange, green, and pink) are bound to the different component proteins, producing a brightly colored ladder with an easy-to- pattern. ™
Spectra Multicolor Broad Range Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
™
• Comprehensive—10 proteins with similar intensity spanning a broad range of 10 to 260 kDa • Versatile—compatible with Coomassie dye staining and western blotting
Thermo Scientific Spectra Multicolor High Range Protein Ladder is a mixture of 8 blue-, green-, and orange-stained proteins for use as size standards for high molecular weight proteins in gel electrophoresis and western blotting. This marker is designed for monitoring the progress of gels during SDS-PAGE, assessing western blot transfer efficiency, and estimating the approximate size of proteins after gel staining or western blotting. ™
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The Spectra Multicolor Broad Range Protein Ladder is recommended for Tris-glycine, Bis-Tris, and Tris-acetate gels.
™
• Comprehensive—8 proteins of similar intensity spanning a range of 40 to 300 kDa; 3 different chromophores (blue, orange, and green) are bound to the different component proteins, producing a brightly colored ladder with an easy-to- pattern
Spectra Multicolor High Range Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The Spectra Multicolor High Range Protein Ladder is recommended for Tris-glycine, Bis-Tris, and Tris-acetate gels.
• Versatile—compatible with Coomassie dye staining and western blotting
Learn more at
Learn more at
thermofisher.com/prestainedstandards
For ordering information refer to page 86.
45
thermofisher.com/prestainedstandards
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
46
Select precast gel
Prepare samples and select buffers
Choose the electrophoresis chamber system and power supply
Select the standard
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
47
Other ladders and standards
MagicMark XP Western Protein Standard kDa
kDa
PageRuler Prestained NIR Protein Ladder
kDa
kDa
460
198
220
98
268 238
62
171
Accurate molecular weight estimation directly on western blots
260 160
120 100
49
117
80
110 80
60
50
60 40
50
38
30
40
28
MagicMark XP Western Protein Standard 20 NuPAGE Bis-Tris gel, blotted to nitrocellulose, 15 and detected with Invitrogen™ WesternBreeze™ 10 Chemiluminescent Kit
71
17
30
14
55
The MagicMark™ XP Western Protein Standard 20 6 3.5 is specifically designed for easy and convenient 41 3 protein molecular weight estimation directly on 31 HiMark SeeBlue MagicMark XP Novex Sharp Standard Pre-stained western blots. Each recombinant protein Plus2 in the Pre-stained Cat. No. LC5800 LC5602 LC5925 LC5699 Storage specifications standard contains an IgG binding site, which NuPAGE 3–8% NuPAGE NuPAGE NuPAGE Bis-Tris Gel, Tris-Acetate Gel blotted to 4–12% Bis-Tris 4–12% Bis-Tris binds the primary or secondary antibody used w/ Tris-acetate • nitrocellulose, Gel w/ MES Gel w/ MES S torage buffer: Tris-HCl (pH 6.8), DTT, glycerol, SDS, and detected w/ SDS buffer SDS buffer WesternBreeze for detection of the target protein, allowing direct SDS buffer Chemiluminescent bromophenol blue Kit visualization of the standard on the western blot. ™
®
®
®
®
™
®
®
®
• Storage conditions: upon receipt store at –20°C
• Comprehensive—consists of 9 recombinant proteins from 20 to 220 kDa • Versatile—compatible with chemiluminescent, chromogenic, and fluorescent detection
• Stability: 4 months from date of receipt
Recommended products The MagicMark XP Western Protein Standard is compatible with a broad range of gels—NuPAGE Bis-Tris gels, Novex Tris-Glycine gels, Novex Tricine gels, NuPAGE Tris-Acetate gels, and Bolt Bis-Tris Plus gels.
Sharp prestained standard for near-IR fluorescent visualization and protein sizing Thermo Scientific™ PageRuler™ Prestained NIR Protein Ladder is a mixture of 10 proteins that are stained blue and labeled with a fluorophore for near-infrared (NIR) fluorescent visualization and protein sizing following electrophoresis. The molecular weight markers in this ladder resolve into sharp bands when analyzed by SDS-PAGE. The 55 kDa band is of greater intensity and serves as a reference band. • Comprehensive—10 protein bands spanning 11 to 250 kDa • Versatile—visualize using instruments equipped for detection of near-infrared fluorescence such as certain Typhoon™ Imagers and the LI-COR Odyssey™ Infrared Imaging System; bands are directly visible because the proteins are prestained blue
Learn more at
Visual detection
Infrared detection
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The PageRuler Prestained NIR Protein Ladder is recommended for visual detection, infrared imaging detection, and western blotting.
Learn more at
thermofisher.com/westernblotstandard
For ordering information refer to page 86.
PageRuler Prestained NIR Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
thermofisher.com/specialtystandards
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
48
Prepare samples and select buffers
Select precast gel
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
BenchMark Fluorescent Protein Standard
BenchMark His-tagged Protein Standard
Efficient estimation of molecular weight by fluorescent detection
Convenient detection and protein sizing of His-tagged proteins
BenchMark Fluorescent Protein Standard NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
The Invitrogen BenchMark Fluorescent Protein Standard consists of Alexa Fluor™ 488 dye– conjugated proteins for molecular weight estimation of fluorescently labeled proteins. ™
Storage specifications
The Invitrogen BenchMark His-tagged Protein Standard can be used as a positive control and for molecular weight sizing in His-tagged fusion protein detection. Each protein in the standard has a 6xHis tag.
• Storage buffer: Tris-HCl, SDS, glycerol, and Coomassie Blue G-250
• Comprehensive—10 sharp and clear bands from 10 to 160 kDa for molecular weight estimation of His-tagged proteins
™
• Comprehensive—consists of 7 distinct protein bands in the range of ~11–155 kDa • Versatile—visualize on a UV transilluminator or laser-based scanning instrument after SDS-PAGE
™
• Storage conditions: upon receipt store at –20°C • Stability: 6 months from date of receipt
Recommended products The BenchMark Fluorescent Protein Standard is recommended for use with NuPAGE gels or Novex Tris-Glycine gels.
BenchMark His-tagged Protein Standard NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
™
• Versatile—can be visualized with Invitrogen™ InVision™ HisTag In-Gel Stain or Coomassie R-250 stain on SDS-PAGE gels, or with Anti-His (C-term) Antibody using chromogenic or chemiluminescent detection systems
49
SimplyBlue stain
InVision stain
Storage specifications • Storage buffer: Tris-HCl, SDS, glycerol, DTT, and Coomassie Blue G-250 • Storage conditions: upon receipt store at –20°C • Stability: 6 months from date of receipt
Recommended products The BenchMark His-tagged Protein Standard is recommended for use with NuPAGE gels and Novex Tris-Glycine gels.
Learn more at thermofisher.com/specialtystandards
Learn more at thermofisher.com/specialtystandards
IEF Marker 3-10 Accurate determination of protein isoelectric points The IEF Marker 3-10 is a ready-to-use protein standard developed for IEF applications. This marker can be used for monitoring of protein separation on IEF gels and pI determination of unknown protein samples. • Comprehensive—13 purified isoforms from pI 3.5–10.7; no additional high range or low range markers are required • Versatile—can be used for both native and denaturing conditions
IEF Marker 3-10 Novex pH 3–10 Gel
Storage specifications • Storage buffer: 10% glycerol containing bromophenol blue (0.01%) and methyl red (0.01%) • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Learn more at
Recommended products
thermofisher.com/iefstandards
The IEF Marker 3-10 is applicable to all IEF gels (vertical or horizontal).
For ordering information refer to page 86.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
50
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
51
Electrophoresis chambers and power supplies Electrophoresis run considerations: In electrical , the process of electrophoresis is closely associated with the following equations derived from Ohm’s Law: Voltage = Current × Resistance (V = IR) Wattage = Current × Voltage (W = IV)
Which electrophoresis chamber system is right for you?
Resistance
Current
The electrical resistance of the assembled electrophoresis cell is dependent on buffer conductivity, gel thickness, temperature, and the number of gels being run. Although the resistance is determined by the gel system, the resistance varies over the course of the run.
For a given gel/buffer system, at a given temperature, current varies in proportion to the field strength (voltage) and crosssectional area (thickness and number of gels). When using a constant current setting, migration starts slow, and accelerates over time, thus favoring stacking in discontinuous gels.
• In discontinuous buffer systems (and to a lesser extent in continuous buffer systems) resistance increases over the course of electrophoresis. This occurs in the Tris-glycine buffer system as highly conductive chloride ions in the gel are replaced by less conductive glycine ions from the running buffer.
When running under constant current, set a voltage limit on the power supply at, or slightly above the maximum expected voltage to avoid unsafe conditions. At constant current voltage increases as resistance increases. If a local fault condition occurs (e.g., a bad connection), high local resistance may cause the voltage to reach the maximum for the power supply, leading to overheating and damage of the electrophoresis cell.
• Resistance decreases as the temperature increases.
Voltage The velocity of an ion in an electric field varies in proportion to the field strength (volts per unit distance). The higher the voltage, the faster an ion moves. For most applications, we recommend a constant voltage setting. • A constant voltage setting allows the current and power to decrease over the course of electrophoresis, providing a safety margin in case of a break in the system. • The constant voltage setting does not need adjustment to for differences in number or thickness of gels being electrophoresed.
For ordering information refer to page 87.
Mini Gel Tank
XCell SureLock Mini-Cell
XCell4 SureLock Midi-Cell
Gel capacity
Up to 2 minigels
Up to 2 minigels (8 x 8 cm)
Up to 4 midigels (8 x 13 cm)
Cell dimensions (L x W x H)
32 x 11.5 x 16 cm (height with lid on)
14 x 13 x 16 cm (height with lid on)
21 x 19 x 16 cm (height with lid on)
Advantages
• The Mini Gel Tank is versatile and compatible with NuPAGE, Bolt, or Novex minigels. The unique tank design enables convenient sideby-side gel loading and enhanced viewing during use. • Mini Blot Module is available for wet protein transfers.
• XCell II Blot Module is available for semi-wet protein transfers • Instrument incorporates a gel tension wedge in place of the rear wedge used on earlier models
• Advanced apparatus for easier, more reliable electrophoresis with midigels
Power Wattage measures the rate of energy conversion, which is manifested as heat generated by the system. Using constant power ensures that the total amount of heat generated by the system remains constant throughout the run, but results in variable mobility since voltage increases and current decreases over the course of the run. Constant power is typically used when using IEF strips. When using constant power, set the voltage limit slightly above the maximum expected for the run. High local resistance can cause a large amount of heat to be generated over a small distance, damaging the electrophoresis cell and gels.
Learn more at thermofisher.com/electrophoresischambers
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
52
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
53
Mini Gel Tank One tank, 181 gels The Mini Gel Tank is designed for more intuitive use and greater convenience compared to traditional electrophoresis tanks (Figure 24). The unique, side-by-side tank design allows you to perform electrophoresis of 1 or 2 minigels.
1. Snap the electrophoresis tank into the base, and place the cassette clamp(s) into the chamber(s) with the anode connector(s) (+) aligned to the center.
2. Remove the comb, and peel away the tape at the bottom of the gel cassette.
3. Place the cassette in the chamber with the wells facing towards you.
Rinse the wells 3 times with 1X buffer.
Hold the cassette in a raised position and close the clamp by moving the cam handle forward.
Fill the chamber(s) with 1X buffer to the level of the cathode.
The Mini Gel Tank offers: • Versatility—compatible with all of our minigels, including NuPAGE, Novex, Bolt, and specialty gels • Easy sample loading—forward-facing well configuration • Simultaneous visualization of both gels—streamlined, side-by-side tank configuration • Simple monitoring of gels—white tank stand provides easy visualization of prestained markers • Less running buffer required—gel chambers are separated, so you only need to load sufficient buffer for each gel to the specified fill line
Specifications • Gel capacity: up to 2 minigels • Cell size (L x W x H): 32 x 11.5 x 16 cm (height with lid on) • Buffer requirement: 400 mL for each minigel chamber • Material: polycarbonate • Chemical resistance: not compatible with acetone, chlorinated hydrocarbons, or aromatic hydrocarbons
4. Make sure the wells are completely filled with 1X buffer. Load your samples and markers.
5. Hold the cassette and release the cassette clamp.
6. Make sure the power supply is off.
Gently lower the casette so that it rests on the bottom of the chamber, and close the cassette clamp.
If only running one gel, remove the cassette clamp from unused chamber.
Add 1X buffer to the level of the fill line.
Place the lid on the tank and plug the electrode cords into the power supply. Turn the power supply on to begin electrophoresis.
Figure 24. How to use the Mini Gel Tank.
Figure 25. Electrophoresis of Bolt gel using the Mini Gel Tank. Protein standards and samples were loaded at 10 µL sample volumes in an Invitrogen™ Bolt™ 4–12% Bis-Tris Plus Gel. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands with consistent migration patterns were observed after staining with SimplyBlue SafeStain. Images were acquired using a flatbed scanner. Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 µg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 µg HeLa cell lysate; Lane 5: 20 µg HeLa cell lysate; Lane 6: 5 µg BSA; Lane 7: 40 µg Jurkat cell lysate; Lane 8: 5 µg GST fusion protein; Lane 9: Novex Sharp Unstained Protein Standard; Lane 10: 5 µg β-galactosidase.
Watch our Mini Gel Tank video. thermofisher.com/minigeltank
Recommended products The Mini Blot Module is a wet transfer device that conveniently fits into the chambers of the Mini Gel Tank to easily transfer proteins from minigels to nitrocellulose or PVDF membranes.
Learn more at thermofisher.com/minigeltank For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
54
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
XCell SureLock Mini-Cell
55
Figure 26. How to use the XCell SureLock Mini-Cell.
Simultaneous electrophoresis of up to 2 minigels The unique design of the Invitrogen™ XCell™ SureLock Mini-Cell allows you to run minigels quickly and easily without any clamps or grease (Figure 26). The tight seal provided by the gel tension wedge results in leak-free, consistent performance. The XCell SureLock Mini-Cell is compatible with NuPAGE, Novex, and specialty gels (Figure 27).
1. Drop buffer core into the lower 2. Lock the gel tension wedge in buffer chamber of the XCell place, load samples, and fill the SureLock Mini-Cell. Insert one buffer chambers with the minigel in front of the buffer core and appropriate running buffers. a second minigel or the buffer dam behind the buffer core.
Key features of the XCell SureLock Mini-Cell:
Specifications
• -friendly design—uses single gel tension wedge with no clamps or grease
• Gel capacity: up to 2 minigels
• Flexibility—perform electrophoresis of 2 minigels simultaneously
• Buffer chamber requirement (Novex minigels):
• Unique, heat dissipating design—no need for a cooling device • Built-in safety features—retractable plugs, recessed jacks, and a specially designed lid enhances safety
• Chemical resistance: The XCell SureLock Mini-Cell is impervious to most alcohols but not compatible with acetone, chlorinated hydrocarbons (e.g., chloroform), or aromatic hydrocarbons (e.g., toluene, benzene)
Learn more at thermofisher.com/surelockmini
For ordering information refer to page 87.
Figure 27. Electrophoresis of NuPAGE Bis-Tris gels with the XCell SureLock Mini-Cell. Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 µg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 µg HeLa cell lysate; Lane 5: 20 µg HeLa cell lysate; Lane 6: not used; Lane 7: 40 µg Jurkat cell lysate; Lane 8: 5 µg of a GST fusion protein; Lane 9: Invitrogen™ Novex™ Sharp Protein Standard; and Lane 10: 5 µg β-galactosidase. Gel electrophoresis was performed at 200 V (constant) and gels were stained using SimplyBlue SafeStain. Images were acquired using a flatbed scanner.
• Cell size (L x W x H): 14 x 13 x 16 cm (height with lid on)
–– Upper buffer chamber: 200 mL –– Lower buffer chamber: 600 mL
3. Place the cell lid on the unit and you’re ready to run.
NuPAGE Bis-Tris gel in XCell SureLock Mini-Cell
Recommended products The XCell SureLock Mini-Cell can be easily adapted for transfer of proteins from minigels to membranes by simply inserting the XCell II Blot Module in the lower buffer chamber.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
56
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
XCell4 SureLock Midi-Cell
A.
Simultaneous electrophoresis of up to 4 midigels The Invitrogen™ XCell4 SureLock™ Midi-Cell allows simultaneous vertical electrophoresis of 1–4 midigels without leaking, enabling consistent performance. The system is designed to dissipate heat effectively and evenly, and enable highresolution results when using Novex midigels (Figure 29).
B.
1. Insert the XCell4 SureLock Assembly in its unlocked position into the center of the Midi-Cell base. The XCell4 SureLock Assembly slides down over the protrusion in the Midi-Cell base.
4. The upper buffer chamber (cathode) is the void formed between a gel and the buffer core at the center of each core.
2. Place one cassette on each side of the buffer core for each of the two cores. For each cassette, the shorter “well” side of the cassette must face out towards the lower buffer chamber.
5. Lock the XCell4 SureLock Assembly by moving the tension lever to the locked position (indicated on the XCell4 SureLock Assembly). This will squeeze the gels and buffer cores together, creating leak-free seals.
3. While holding the assembly together with your hands (A), insert the buffer cores with the gel cassettes into the lower buffer chamber such that the negative electrode fits into the opening in the gold plate on the lower buffer chamber (B). Always hold the cassette assembly by its edges as shown in the figure.
6. Proceed to loading samples and buffers.
Note: If you are having difficulty inserting the assembly into the lower buffer chamber, make sure the cathode (black polarity indicator) of the buffer core is aligned with the cathode (black polarity indicator) of the lower buffer chamber.
Specifications Key features of the XCell4 SureLock Midi-Cell: • -friendly design—leak-free electrophoresis without clamps or grease • Flexibility—perform electrophoresis of 1–4 midigels • Unique, heat dissipating design—no need for a cooling device • Built-in safety features—specially designed lid enhances safety
57
• Gel capacity: up to 4 midigels (8 x 13 cm) • Cell size (L x W x H): 21 x 19 x 16 cm (height with lid on) • Buffer chamber requirement: –– Upper buffer chamber: 175 mL x 4 –– Lower buffer chamber: 540–700 mL • Chemical resistance: not compatible with acetone, chlorinated hydrocarbons, or aromatic hydrocarbons
Figure 28. How to use the XCell4 SureLock Midi-Cell with 4 gels.
1
2
3
4
5
6
7
8
9
10 11
12
13
14
15
16
17
18
19 20
Figure 29. Quality of a precast NuPAGE Novex 4–12% Bis-Tris Midi Gel with a variety of protein standards, lysates and purified proteins. Electrophoresis was performed using MES running buffer and an XCell4 SureLock Midi Cell at 200 V (constant). Following electrophoresis, the gel was stained using SimplyBlue SafeStain, destained using water, and imaged using a flatbed scanner. Sharp, straight bands were observed. Lanes 1, 10, 11, and 20 were each loaded with 5 µL of Mark12 Unstained Standard (blend of 12 purified proteins). Lanes 2, 9, 12, and 19 were each loaded with 10 µg of E. coli lysate. Lanes 3 and 18 were each loaded with 6 µg of human IgG. Lanes 4 and 17 were each loaded with 6 µg of human IgM. Lanes 5 and 16 were each loaded with 5 µL of SeeBlue Plus2 Prestained Protein Standard. Lanes 6 and 15 were each loaded with 5 µL of BenchMark Protein Ladder. Lanes 7 and 14 were each loaded with 15 µL of MagicMark XP Western Protein Standard. Lanes 8 and 13 were each loaded with 5 µL of HiMark Unstained Protein Standard.
Learn more at thermofisher.com/surelockmidi For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
58
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Post stain
Select precast gel
Prepare samples and select buffers
Choose the electrophoresis chamber system and power supply
Select the standard
Stain the gel
Run the gel
Post stain
59
Run the gel PowerEase 90W Power Supply
Table 4. Gel running conditions in electrophoresis chamber systems. Running conditions in XCell Surelock Mini-Cell
Simple, affordable power supply specifically for minigel electrophoresis
Bolt 4–12% (MES)
The Invitrogen™ PowerEase™ 90W Power Supply is designed specifically for minigel electrophoresis. The straightforward, intuitive interface makes the powering of gel runs a simple and easy process. In addition, the PowerEase 90W Power Supply features: • Constant voltage or current settings • Built-in timer for walk-away gel electrophoresis • Output jacks that are compatible with most electrophoresis devices
PowerEase 300W Power Supply
Voltage (V)
Starting current (mA)*
End current (mA)*
Approximate run time (minutes)
NA
NA
NA
NA
Running conditions in Mini Gel Tank
Voltage (V)
Starting current (mA)*
Approximate End current run time (mA)* (minutes)
200
160
70
20
Bolt 4–12% (MOPS)
NA
NA
NA
NA
200
160
50
35
NuPAGE 4–12% Bis-Tris (MES)
200
100 to 125
60 to 80
35
200
160
90
30
NuPAGE 4–12% Bis-Tris (MOPS)
200
100 to 125
60 to 80
50
200
140
50
42
Novex 4–20% Tris-Glycine (denatured)
125
30 to 40
8 to 12
90
125
40
10
100
Novex 4–20% Tris-Glycine (native)
125
6 to 12
3 to 6
1 to 12 hours
125
30
10
90
NuPAGE 3–8% Tris-Acetate (denatured)
150
40 to 55
25 to 40
60
150
60
20
50
NuPAGE 3–8% Tris-Acetate (native)
150
18
7
2 to 3 hours
150
40
10
100
Novex 10–20% Tricine
125
80
40
90
125
110
40
65
NativePAGE 3–12%
150
12 to 16
2 to 4
90 to 115
150
10
<10
80
pH 3-10 IEF
10% Zymogram (Gelatin)
100
7
NA
60
100
8
NA
60
200
NA
NA
60
200
NA
NA
60
500
NA
5
30
500
NA
5
30
125
30 to 40
8 to 12
90
125
40
10
90
* Per gel. Note: Run times may vary depending on the power supply and gel percentage.
Learn more at thermofisher.com/powerease
Programmable power supply designed for high-throughput gel electrophoresis The Invitrogen™ PowerEase™ 300W Power Supply is a fully programmable power supply designed for high-throughput gel electrophoresis. The straightforward, intuitive interface makes the powering of gel runs a simple and easy process. In addition, the PowerEase 300W Power Supply features: • Constant voltage, current, or power settings • Built-in timer for walk-away gel electrophoresis • Up to 10 custom programs with 10 steps each • Four sets of output jacks that are compatible with most electrophoresis devices
For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
60
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
61
Troubleshooting tips XCell SureLock Mini-Cell troubleshooting
Electrophoresis troubleshooting
Observation
Cause
Solution
Problem
Possible cause
Suggested solution
Run taking longer than usual
Buffers are too dilute
Check if buffer was diluted properly. Check buffer recipe; dilute from concentrate or remake if necessary.
Run taking longer time with recommended voltage
Running buffer too dilute
Make fresh running buffer and use a 1X dilution.
Upper buffer chamber is leaking
Make sure the buffer core is firmly seated, the gaskets are in place and the gel tension lever is locked.
Running buffer too concentrated
Make fresh running buffer and use a 1X dilution.
Voltage is set too low
Set correct voltage.
Current too high and excessive heat generated with recommended voltage
Remove tape from bottom of cassette.
Current too low or no current with recommended voltage
Incomplete circuit
Tape left on the bottom of the cassette Connection to power supply not complete
Check all connections with a voltmeter for conductance.
Remove the tape from the bottom of the gel cassette prior to electrophoresis. Make sure the buffer covers sample wells; check the wire connections on the buffer core.
Insufficient buffer level
Make sure the upper buffer (cathode) is covering the wells of the gel. Be sure there is sufficient buffer in the Lower Buffer Chamber to cover the slot at the bottom of the gel.
Streaking of proteins
Sample overload
Load less protein.
High salt concentration in sample
Decrease the sample salt concentration by dialysis or gel filtration.
Sample precipitates
Increase the concentration of SDS in the sample.
Contaminants such as lipids or DNA complexes in sample
Centrifuge or clarify the sample to remove particulate contaminants. Treat sample with nuclease(s).
Poorly poured gel
Make sure the gel is poured evenly and all at once.
Protein sample only partially denatured
Fully denature the protein.
Protein sample only partially reduced
Make sure a sufficient amount of DTT or β-mercaptoethanol is added.
Gel runs for too long
Watch the dye front as an indicator for proper running time.
Loading a large volume of sample causes incomplete stacking
Load appropriate volume of sample. If the sample is too dilute, concentrate it using ultrafiltration.
Uneven electric field during run
Try to make sure the loading is symmetrical if the protein concentration is known.
Uneven surface of the resolving gel
Try to make the resolving gel surface even while pouring the gel.
Expired gels
Use the gels before the specified expiration date; Note: NuPAGE gels have an extended 12 month shelf life, minimizing the risk of having expired gels.
Current reading on power supply is zero or very low
Run is faster than normal with Buffers are too concentrated poor resolution or incorrect
Cannot see the sample wells to load sample
Check buffer recipe; dilute or re-make if necessary.
Voltage, current, or wattage is set at a higher limit
Decrease power conditions to recommended running conditions (see page 59).
There is little contrast between the sample well and the rest of the gel
Mark cassette at the bottom of the wells with a marker pen prior to assembling the Upper Buffer Chamber. Illuminate the bench area with a light source placed directly behind the XCell SureLock unit.
Fuzzy bands
Mini Gel Tank troubleshooting Observation
Cause
Solution
Run taking longer than usual
Buffers are too dilute
Check buffer recipe; dilute from concentrate or remake if necessary.
Buffer chamber is leaking
Make sure the cassette clamp is firmly seated, the gaskets are in place and the cassette clamp is locked.
Current is set too low
Set correct current.
Tape left on the bottom of the cassette
Remove tape from bottom of cassette.
Connection to power supply not complete
Check all connections with a voltmeter for conductance.
Insufficient buffer level
Make sure there is sufficient buffer in the electrophoresis tank to cover the wells of the gel.
Current reading on power supply is zero or very low
Run is faster than normal with Buffers are too concentrated poor resolution or incorrect
Cannot see the sample wells to load sample
Dumbbell shaped bands or “smiling” bands
Check buffer recipe; dilute or re-make if necessary.
Current is set at a higher limit
Decrease current to recommended running conditions (see page 59).
There is little contrast between the sample well and the rest of the gel
Mark cassette at the bottom of the wells with a marker pen prior to placing the cassette in the electrophoresis tank.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
62
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Stain the gel
Run the gel
Stain the gel
Protein stains
Silver stains
Once protein bands have been separated by electrophoresis, they can be directly visualized using different methods of in-gel detection. Over the past several decades, demands for improved sensitivity for small sample sizes and compatibility with downstream applications and detection instrumentation have driven the development of several basic staining methods. Each method has particular advantages and disadvantages, and a number of specific formulations of each type of method provide optimal performance for various situations.
• Invitrogen™ SilverXpress™ Silver Stain
Protein gel electrophoresis technical handbook
Post stain
Coomassie dye protein gel stains
• Thermo Scientific™ Pierce™ Silver Stain
• Thermo Scientific™ Pierce™ Silver Stain for Mass Spectrometry
Fluorescent/specialty stains
Coomassie stains
• Pierce Reversible stain for nitrocellulose or PVDF membranes • Pro-Q™ Emerald Glycoprotein stain • Pro-Q™ Diamond Phosphoprotein stain To visualize the proteins, a protein-specific, dye-binding or color-producing chemical reaction must be performed on the
™
• SimplyBlue SafeStain • Thermo Scientific™ Imperial™ Protein Stain
The most common methods for in-gel protein detection use stains with Coomassie dye. These stains use either the G-250 (colloidal) or R-250 form of the dye (Table 6). Colloidal Coomassie stain can be formulated to effectively stain proteins within one hour and require only water (no methanol or acetic acid) for destaining.
of the stain, various steps are necessary to hold the proteins in the matrix and to facilitate the necessary chemical reaction. Most staining methods involve some version of the same general incubation steps: S
DI H2O
DI H2O
shown for SimplyBlue SafeStain (Figure 30, 33 and 34), PageBlue (Figure 31 and 35).
• Simple—Coomassie dye–based formulations are easy to formulate and are widely used
Learn more at thermofisher.com/coomassiestains
• Economical—Coomassie dye–based stain formulations are cost effective 1. Water wash.
2. Fix.
3. Water wash.
4. Stain.
5. Destain.
• A water-wash to remove electrophoresis buffers from the gel matrix • An acid or alcohol wash to condition or fix the gel to limit diffusion of protein bands from the matrix
• Flexible—useful for qualitative visualization, quantitative densitometry, and gel excision and analysis by mass spectrometry Table 6. Coomassie dye–based protein gel stains. SimplyBlue SafeStain
Imperial Protein Stain
PageBlue Protein Staining Solution
Type
G-250
R-250
G-250
Limit of detection
>7 ng
3 ng
5 ng
Time to stain (min)
12
60
60
Depending on the particular staining method, two or more of
Compatible with: PVDF membranes Nitrocellulose membranes
Yes No
Yes No
Yes No
these functions can be accomplished with one step. For example,
Reusable
No
No
Yes (up to 3x)
fix and stain in one step. Conversely, certain functions require
Mass spectrometry compatible
Yes
Yes
Yes
several steps. For example, silver staining requires both a
Color
Purple
Purple
Blue-green
Feature
Free of methanol and acetic acid
Photographs better than Coomassie G-250 dye
Free of methanol and acetic acid
Advantages
Rapid, sensitive completely non-hazardous (does not require methanol or acetic acid fixatives or destains) staining
Fast, ultrasensitive protein detection
Cost-effective option for fast, sensitive staining
• Treatment with the stain reagent to allow the dye or chemical to diffuse into the gel and bind (or react with) the proteins • Destaining to remove excess dye from the background gel matrix
staining reagent step and a developer step to produce the colored reaction product.
Learn more at thermofisher.com/proteinstains For ordering information refer to page 87.
with simplified protocols. Example data and staining protocols are
Key features:
• Easy to use—simply soak the gel in stain solution and destain to observe protein bands
S
Our Coomassie stains provide sensitive protein detection along
Protein Staining Solution (Figure 32), and Imperial Protein Stain
proteins within the gel. Depending on the particular chemistry
a dye reagent that is formulated in an acidic buffer can effectively
• Thermo Scientific PageBlue stain ™
Convenient, ready-to-use reagents with no permanent chemical modification
• Invitrogen™ SYPRO™ Orange, Red, or Ruby gel stain
Typically these stains can be classified broadly based on the dye or molecule that helps visualize the protein stains:
63
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
64
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Protocols
65
Example data 1 2 3 4 5 6 7 8 9 10 2
3
1
DI H2O
S
DI H2O
DI H2O
Figure 30. SimplyBlue SafeStain protocol.
1. Wash the gel three times (5 minutes) with ultrapure water.
2. Add SimplyBlue SafeStain (1 hour).
3. Wash gel with 100 mL of DI water for 1 hour.
Figure 33. Sensitive staining results with SimplyBlue SafeStain. The following samples were separated on a NuPAGE Novex 4-12% Bis-Tris gel and then stained with SimplyBlue SafeStain. Lane 1: 6 µg protein mix; Lane 2: 1 µg rabbit IgG; Lane 3: 1 µg reduced BSA; Lane 4: 5 µg E. coli lysate; Lane 5: 20 ng reduced BSA; Lane 6: 10 ng reduced BSA; Lane 7: 7 ng reduced BSA; Lane 8: 3 ng reduced BSA; Lane 9: 10 µL Mark12 Unstained Standard (blend of 12 purified proteins); Lane 10: 5 µL Mark12 Unstained Standard.
4. Additional water wash with 100 mL of DI water (1 hour) for increased sensitivity.
1
3 4
5
6
7
8
9
1
2
3 4
5
6
7
8
9
1
2
3 4
5
6
7
8
9
Rabbit IgG
S
DI H2O
2
Figure 34. Two-dimensional electrophoresis (2DE) analysis of spinach chloroplast extract; staining with SimplyBlue SafeStain. Spinach chloroplast extract was prefractionated in the ZOOM™ IEF Fractionator and the individual fractions were then separated by 2DE using narrow pH range ZOOM™ Strips and NuPAGE™ Novex 4–12% Bis-Tris ZOOM™ Gels. Gels were Coomassie stained using SimplyBlue SafeStain.
DI H2O BSA Protein A
Figure 31. Imperial Protein Stain protocol.
1. Wash the gel three times with deionized water (15 minutes).
2. Add Imperial Protein Stain (5 minutes−1 hour).
3. Water destain (15 minutes−overnight).
Protein G Lysozyme
5-minute stain; 15-minute water destain
1-hour stain; 2-hour water destain
1-hour stain; overnight water destain
Figure 35. Enhanced sensitivity and clear background using Imperial Protein Stain. For even greater sensitivity and reduced background, gels can be stained with Imperial Protein Stain for 1 hour and washed with water from 1 hour to overnight. Lane 1: BSA only (6 µg); Lane 2–9: loaded left to right with 1,000, 200, 100, 50, 25, 12, 6, and 3 ng protein sample.
Did you know? DI H2O
S
DI H2O
Staining with a Coomassie stain prior to silver staining allows for more uniform staining of certain proteins since silver ions can interact with certain functional groups such as carboxylic acid groups, imidazole, sulfhydryls, and amines.
DI H2O
Recommended products
1. Wash the gel three times with ultrapure water (30 minutes).
2. Add PageBlue Protein Staining Solution (1 hour).
For ordering information refer to page 87.
3. Rinse gel two times with ultrapure water (<1 minute)
4. Wash gel one time with ultrapure water (5 minutes)
Figure 32. PageBlue Protein Staining Solution protocol.
The Thermo Scientific Pierce Power Stainer is designed for rapid Coomassie dye staining of proteins in up to two minigels and subsequent removal of unbound stain from the gel in a single step. Refer to page 72 of this brochure for more information.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
66
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Silver stains
We offer highly sensitive silver stains with short protocol times that are also compatible with mass spectrometry (Table 7). The SilverXpress™ Silver Staining Kit provides nanogram-level sensitivity
Pierce Silver Stain for Mass Spectrometry
Pierce Silver Stain Kit
SilverXpress Silver Staining Kit
Components (steps)
6 (17)
4 (15)
5 (13)
Time required
1 hr 13 min
2 hr 25 min
2 hr
Limit of detection
0.25 ng
0.25 ng
0.86 ng
Mass spectrometry compatible
Yes
Yes
Yes
Storage
Room temperature
Room temperature
4°C
Stability
1 year
1 year
6 months
Advantages
• Fast and sensitive staining and destaining of protein gels • Optimized for peptide recovery after in-gel typsin digestion for mass spectrometry • Flexible gel fixation (15–30 min to overnight) and staining (1–30 min)
• Rapid, ultrasensitive and versatile silver stain system • Flexible gel fixation (30 min to overnight) and staining (5 min to 20 hours)
• Nanogram-level sensitivity for silver staining with minimal background
Protocols and example data
with minimal background (Figure 37), while the Pierce™ Silver Stain Kit provides protocol flexibility (Figure 38 and 39).
SZ
F
H2O
1 2 3 4 5 6 7 8 9 10
Key features: • Sensitive—silver stains are highly sensitive stains that allow for visualization of proteins at sub-nanogram levels
Learn more at thermofisher.com/silverstains 1. Wash the gel with water.
• Easy to use and flexible—optimized for minimal steps and flexibility to accommodate shorter or longer protocols • Workflow compatible—our mild chemical formulations help ensure compatibility with mass spectrometry and sequencing • Robust performance—detailed protocol enables consistent results with clear background
67
Table 7. Silver stain kits.
Ultra-sensitive stains with optimized protocols, manufactured for minimal variability Silver staining is the most sensitive colorimetric method for detecting total protein, and functions by the deposition of metallic silver at the location of protein bands. Silver ions (from silver nitrate in the stain reagent) interact and bind with certain protein functional groups. The strongest interactions occur with carboxylic acid groups (Asp and Glu), imidazole (His), sulfhydryl groups (Cys), and amines (Lys). Various sensitizer and enhancer reagents are essential for controlling the specificity and efficiency of silver ion binding to proteins and effective conversion (development) of the bound silver to metallic silver.
Protein gel electrophoresis technical handbook
Post stain
2. Fix the gel in Fixing Solution for 10 minutes.
3. Decant the Fixing Solution and incubate the gel in 2 changes of Sensitizing Solution. S
H2 O S 4. Decant the Sensitizing Solution and rinse the gel two times with ultrapure water.
5. Incubate the gel in Staining Solution.
D
H2O
D 6. Decant the Staining Solution and rinse the gel two times with ultrapure water.
7. Incubate the gel in Developing Solution.
SS
Figure 37. Crystal clear background with the SilverXpress Kit. Samples were separated on a NuPAGE Novex 4-12% Bis-Tris gel and stained with the SilverXpress Kit. Lanes 1, 10: Mark12 Unstained Standard (blend of 12 purified proteins) diluted 1:4; Lane 2: Mark12 Unstained Standard diluted 1:8; Lane 3: Mark12 Unstained Standard diluted 1:16; Lane 4: Mark12 Unstained Standard diluted 1:32; Lane 5: Mark12 Unstained Standard diluted 1:64; Lane 6: 1.6 ng BSA; Lane 7: 0.8 ng BSA; Lane 8: E. coli lysate diluted 1:20; Lane 9: E. coli lysate diluted 1:80.
H 2O
8. Add the Stopping Solution directly to the gel when the desired staining intensity is reached.
9. Decant the Stopping Solution and wash the gel three times with ultrapure water.
Figure 36. SilverXpress Silver Staining Kit protocol.
For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
68
Prepare samples and select buffers
Select precast gel
Select the standard
Acetic Acid
EtOH H2O
Choose the electrophoresis chamber system and power supply
Run the gel
Lysate staining
Stain the gel
MW marker staining
F
2. Fix 2 x 15 minutes in EtOH/ acetic acid.
SZ
10% EtOH
SZ 3. Incubate 2 x 5 minutes with 10% EtOH. Wash.
4. Mix Sensitizer. Sensitize for 1 minute. Wash 2 x 1 minute.
Fluorescent gel stains are designed for use in 1D and 2D PAGE and offer sensitivities similar to that obtained with silver staining techniques. Invitrogen™ SYPRO™ protein stains are easy-to-use fluorescent stains for the detection of proteins separated by PAGE (Table 8). Stained proteins can be viewed with a standard UV or blue-light transilluminator or with a laser scanner.
thermofisher.com/fluorescentstains Recommended products For optimal sensitivity with Polaroid™ film, SYPRO™ Photographic Filter is recommended.
• Simple—no destaining or timed steps required; minimal hands-on time
5. Mix Silver Stain. Stain for 5 minutes. Wash 2 x 20 seconds.
5% Acetic Acid
• Quantitative—linear quantitation range over two orders of magnitude with low protein-to-protein variability • Highly sensitive—typically more sensitive than Coomassie dye–based stains and equivalent to silver stains
D 7. Remove developer. Stop with 5% Acetic Acid for 10 minutes.
Table 8. SYPRO protein stains.
Figure 38. Pierce Silver Stain Kit protocol.
For ordering information refer to page 87.
Learn more at
Features:
S
6. Mix Developer. Develop for 2-3 minutes.
2 minutes, 30 seconds
Development Time
Figure 39. Pierce Silver Stain Kit exhibits excellent senstivity. In standard minigels, proteins are detectable at greater than 0.25 ng per band or spot.
S
D
2 minutes, 30 seconds
69
Fluorescent protein gel stains Rapid, highly sensitive fluorescent stains for total protein detection after electrophoresis
F 1. Wash 2 x 5 minutes with ultrapure water.
Protein gel electrophoresis technical handbook
Post stain
SYPRO Ruby stain
SYPRO Orange stain
SYPRO Red stain
Limit of detection
0.25 ng
4–8 ng
4–8 ng
Stain and destain time
90 min microwave; 18 hr standard
~1 hr
~1 hr
Ex/Em
280 nm, 450/610 nm
300 nm, 470/510 nm
300 nm, 550/630 nm
Ease of use
Ready to use
Supplied as stock solution
Supplied as stock solution
Compatible applications
Mass spectrometry, IEF, 2D gels, on-membrane staining
Mass spectrometry, IEF, 2D gels, on-membrane staining
Mass spectrometry, IEF, 2D gels, on-membrane staining
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
70
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
71
Stain the gel
Specialty protein stains
Electrophoretic staining technology—Pierce Power Stainer
Our specialty protein stains include in-gel phosphoprotein and glycoprotein detection and on-membrane reversible protein staining kits (Table 9).
Learn more at thermofisher.com/specialtystains
Table 9. Specialty protein stains. Pro-Q Emerald 488 Glycoprotein Gel and Blot Stain Kit
Pro-Q Emerald 300 Glycoprotein Gel and Blot Stain Kit
Pro-Q Diamond Phosphoprotein Gel Staining Kit
Detects
Glycoproteins
Glycoproteins
Phosphoproteins
Sensitivity
4 ng glycoprotein per band
0.5 ng glycoprotein per band
1–16 ng phosphoprotein per band
Stain and destain time
~6 hr
~5 hr
4–5 hr
Ex/Em
510/520 nm
280/530 nm
555/580 nm
Advantages
Selective staining of glycoproteins
Selective staining of glycoproteins
Selective staining of phosphoproteins
The Thermo Scientific Pierce Power Stainer consists of a Thermo Scientific™ Pierce™ Power Station with activated Staining Software and a Thermo Scientific™ Pierce™ Power Stain Cassette. It is designed for rapid Coomassie staining and destaining of proteins in polyacrylamide gels. Traditional Coomassie staining techniques require one hour to overnight staining and destaining to achieve desired results. When used in conjunction with Thermo Scientific™ Pierce™ Midi and Mini Gel Power Staining Kits, the Pierce Power Stainer is designed to provide staining efficiency in as few as 6 minutes that is equivalent to, or better than, traditional Coomassie staining techniques.
Good to know
How does electrostaining work? Cathode (–)
Coomassie staining pad Pre-run and pre-washed SDS-PAGE gel Destaining pad
Anode (+)
Pierce Power Stain Cassette Cathode (–) Staining pad Gel Destaining pad Anode (+)
The significant reduction in protein staining time is accomplished by utilizing inonic Power Stain Solution and Destain Solution to electrophoretically drive the negatively charged Coomassie R-250 dye out of the top gel pad, through the polyacrylamide gel matrix and the bottom gel pad, and toward the positively charged anode.
Watch our Pierce Power Stainer video. thermofisher.com/powerstainer
For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
72
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
73
Pierce Power Stainer Rapid Coomassie dye staining and destaining in approximately 10 minutes The Thermo Scientific™ Pierce™ Power Stainer is designed for rapid Coomassie dye staining of proteins in polyacrylamide gels and subsequent removal of unbound stains to give sharply stained protein bands with minimal or no background. The Pierce Power Stainer offers:
Specifications
• Speed—Coomassie dye staining and destaining of proteins in about 10 minutes
• Mode of transfer: semi-dry blotting
• Convenience—simultaneously stain and destain 1–2 minigels or 1 midigel • Reliable performance—enables staining results that are equivalent to traditional staining techniques • Easy touch programming—intuitive LCD touch-screen interface includes preprogrammed protocols
Recommended products
• Gel compatibility: SDS-PAGE gels
The Pierce Power System can be used both for fast Coomassie dye staining of protein gels and for rapid semi-dry transfer of proteins from gel to membrane. The Pierce Power Stainer can be upgraded by adding the Pierce™ Power Blot Cassette to make a fully functional Pierce Power System with blotting and staining capabilities.
• Running dimension: horizontal • Platform: Pierce™ Power System
Pierce Power Stainer
Conventional Coomassie stain
Did you know? Conventional Coomassie dye–based staining techniques require 1 hour to overnight incubation.
Total time: 11 minutes 1. Wash gel 1 × 5 minutes in water 2. Power Stain/Destain gel, 6 minutes
Total time: 230 minutes to overnight 1. Wash gel 3 × 10 minutes in water 2. Incubate gel in Coomassie stain solution* for 60 minutes 3. Wash gel 2 × 10 minutes in water 4. Destain gel in destaining solution** for 3 × 20 minutes 5. Incubate gel in water for 60 minutes to overnight
Thermo Scientific Pierce Power Stainer
Thermo Scientific Pierce Power Blotter
*Coomassie stain solution: 45% methanol, 10% acetic acid, 0.25% Coomassie R-250 **Destain solution: 30% ethanol, 5% acetic acid
Figure 40. Pierce Power Stainer saves time and maintains sensitivity.
Learn more at thermofisher.com/powerstainer For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
74
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook 75 75
Post stain
Western blotting Transfer and Detection
After electrophoresis, the separated proteins are transferred or blotted onto a second matrix, generally a nitrocellulose or polyvinylidene difluoride (PVDF) membrane. Next, the membrane is blocked to minimize potential nonspecific binding of antibodies to the surface of the membrane. Detailed procedures vary widely for the detection steps of the western blot workflow. One common variation involves direct vs. indirect detection methods. In both the direct and indirect detection methods, the blocked membrane is probed with an antibody (primary antibody) specific to the protein of interest (antigen). In direct detection techniques, this antibody is enzyme conjugated or labeled with a fluorophore. However, in indirect detection techniques, the blocked membrane is probed first with an antibody (primary antibody) which is specific to the antigen followed by another antibody (secondary antibody) raised against the host species of the primary antibody. This secondary antibody is often enzyme conjugated or labeled with a fluorophore. The direct method is not widely used as most researchers prefer the indirect detection method for a variety of reasons.
Horseradish peroxidase (HRP) or alkaline phosphatase (AP) are the most popular enzymes conjugated to antibodies used in the western blot workflow. After incubating the membrane with the detection antibody or antibodies, if an enzyme-conjugated antibody was utilized, an appropriate substrate (chromogenic or chemiluminescent) is added and that results in a detectable product. A popular substrate of choice is a chemiluminescent substrate that, when combined with the enzyme, produces light as a byproduct. With the chemiluminescent substrate, the light output can be captured on film or CCD camera. In recent years fluorescent detection became a popular alternative to the enzymatic detection since it allows for more quantitative data analysis. Fluorescent detection utilizes dye-labeled primary antibodies or dyelabeled secondary antibodies and the signal output is captured on an appropriate imaging system. Whatever substrate is used, the intensity of the signal should correlate with the abundance of the antigen on the blotting membrane.
Key products for western blot transfer: Wet
Semi-dry
Dry
Mini Blot Module
Thermo Scientific Pierce Power Blotter
iBlot™ 2 Dry Blotting System
Key products for western blot detection include: Automated detection
Manual detection Blocking buffers Wash buffers Detergents Enhancers Substrates Stripping buffers X-ray film
We offer a wide range of reagents, kits, equipment, and antibodies to facilitate every step of western blot analysis. iBind™ Flex Western Device
Learn more at thermofisher.com/western
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
76
Protein gel electrophoresis technical handbook
Appendix
77
Protocol quick reference QUICK REFERENCE
QUICK REFERENCE
NuPAGE Bis-Tris Midi Gels
Bolt™ Mini Gels
Bolt™ Mini Gels
Instructions for performing electrophoresis using Bolt ™ Mini Gels are described below.
Prepare gel 1. Cut open the gel cassette pouch and remove the cassette. 2. Remove the gel comb and rinse wells 3 times with 1X Running Buffer. and tank
Prepare samples
Reagent
3. Remove the tape covering the slot at the lower portion of the cassette.
Reduced Sample Non-reduced Sample
Sample Bolt ™ LDS Sample Buffer (4X) Bolt ™ Reducing Agent (10X) Deionized Water
x µL 10 µL 4 µL to 26 µL
x µL 10 µL — to 30 µL
Load samples
Total Volume 40 µL* 40 µL* Heat samples at 70˚C for 10 minutes. * Scale samples up or down by adjusting all volumes proportionally.
Prepare 1X Buffer Run conditions
Each chamber of the tank requires 400 mL of 1X SDS Running Buffer (mix 20 mL of 20X Bolt ™ MES or MOPS SDS Running Buffer with 380 mL of deionized water). The same buffer type must be used for both chambers.
1–4
Run Bolt™ Mini Gels at constant voltage (1 or 2 mini gels). Running Buffer
Standard Run
Run Time*
MES
200 V
22 min
MOPS
200 V
32 min
Prepare Samples
5–7
Cathode
QUICK REFERENCE
NuPAGE Tris-Acetate Mini Gels
NuPAGE Bis-Tris Mini Gels Reduced Sampl e
Sam pl e NuPAGE LDS Sample Buffer (4X) NuPAGE Reducing Agent (10X) Deionized Water Tot al Vol u m e
x μL 2.5 μL 1 μL to 6.5 μL 10 μ L
Non- r educed Sampl e
Load Buffer
Run
10 μ L
Conditions
Add 50 mL 20X NuPAGE MES or MOPS SDS Running Buffer to 950 mL deionized water to prepare 1X SDS Running Buffer. Load the appropriate concentration of your protein sample on the gel. Fill the Upper (200 mL) and Lower (600 mL) Buffer Chambers with the appropriate 1X Running Buffer. For reduced samples, use 200 mL 1X Running Buffer with 500 μL NuPAGE Antioxidant in the Upper Buffer Chamber. Voltage: Run Time: Expected Current:
Prepare Samples
x μL 2.5 μL -to 7.5 μL
Heat samples at 70˚C for 10 minutes.
Load Sample
Denaturing Sample x µL 2.5 µL — 1 µL to 10 µL final
Native Sample x µL — 5 µL — to 10 µL final
*For reduced samples only.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Add Buffer
Fill Upper Buffer Chamber with 175 mL 1X NuPAGE SDS Running Buffer. For reduced samples, use 175 mL 1X NuPAGE SDS Running Buffer with 435 μL NuPAGE Antioxidant in the Upper Buffer Chamber. Add a sufficient volume of 1X NuPAGE SDS Running Buffer to the Lower Buffer Chamber.
Run
Voltage: Run Time: Expected Current:
200 V constant 40 min (MES Buffer), 55 min (MOPS Buffer) 160–200 mA/gel (start); 120–170 mA/gel (end)
Prepare 1X Buffer
Denaturing samples: Add 50 mL 20X NuPAGE Tris-Acetate SDS Running Buffer to 950 mL deionized water. Native samples: Add 100 mL 10X Tris-Glycine Native Running Buffer to 900 mL deionized water.
Load Sample Load the appropriate concentration of your protein sample on the gel. Add Buffer
Fill Upper Buffer Chamber with 175 mL of the appropriate 1X Running Buffer. For reduced samples, use 175 mL 1X Running Buffer with 435 μL NuPAGE Antioxidant in the Upper Buffer Chamber. Add a sufficient volume of Running Buffer the Lower Buffer Chamber.
Run Conditions
Voltage: Run Time: Expected Current:
150 V constant 70 min (denaturing gel), 2–3 hours (native gel) 70–90 mA/gel (start); 50–60 mA/gel (end); (denaturing gel) 40–45 mA/gel (start); 15–20 mA/gel (end); (native gel)
QUICK REFERENCE
Instructions for electrophoresis using the XCell SureLock Mini-Cell are described below.
Prepare 1X Buffer
Sample NuPAGE LDS Sample Buffer (4X) Tris-Glycine Native Sample Buffer (2X) NuPAGE Reducing Agent (10X)* Deionized Water
Heat denaturing samples at 70˚C for 10 minutes. Do not heat native samples.
Add 50 mL 20X NuPAGE MES or MOPS SDS Running Buffer to 950 mL deionized water to prepare 1X SDS Running Buffer.
Conditions
Reagent
For Research Use Only. Not for use in diagnostic procedures.
For research use only. Not for use in diagnostic procedures.
Reagent
x µL 2.5 µL 1 µL to 10 µL final
Non-reduced sample x µL 2.5 µL — to 10 µL final
Prepare 1X Buffer
* Run times may vary depending upon gel type and power supply.
Prepare Samples
Reduced sample
Prepare Samples
Heat samples at 70˚C for 10 minutes.
Fill Line
Cathode
Reagent Sample NuPAGE LDS Sample Buffer (4X) NuPAGE Reducing Agent (10X) Deionized Water
1. Pre-fill the chamber with 1X Running Buffer to the level of the cathode. 2. Place the cassette in the chamber with the wells facing towards you. Hold the cassette in a raised position and close the cassette clamp. 3. Fill all wells with 1X Running Buffer. 4. Load your samples and markers. 5. Hold the cassette and release the cassette clamp. 6. Gently lower the cassette to the bottom of the chamber, and close the cassette clamp 7. Add 1X buffer to the level of the fill line.
NuPAGE Tris-Acetate Midi Gels
Instructions for electrophoresis of Bis-Tris Gels using the XCell4 SureLock Midi-Cell are described below.
200 V constant 35 minutes (MES Buffer), 50 minutes (MOPS Buffer) 100–125 mA/gel (start); 60–80 mA/gel (end)
Intended Use: For research use only. Not for human or animal therapeutic or diagnostic use.
Prepare 1X Buffer
Reagent Denatur i ng Sampl e* Nati ve Sampl e Sam pl e x μL x μL NuPAGE LDS Sample Buffer (4X) 2.5 μL -Tris-Glycine Native Sample Buffer (2X) -5 μL D ei on i z ed Water t o 7.5 μ L to 5 μL Tot al Vol u m e 10 μ L 10 μ L Samples Heat samples at 70˚C for 10 minutes Do not heat *For reduced samples, add NuPAGE Reducing Agent (10X) to 1X.
Novex Tris-Glycine Mini Gels
Novex Tris-Glycine Mini Gels
Instructions are provided below for electrophoresis of NovexTris-Glycine Gels using the XCellSureLock Mini-Cell.
Non-Denaturing (Native) Electrophoresis
Denaturing Electrophoresis
Prepare Samples
Reagent Sampl e Sample x μL Tris-Glycine Native Sample Buffer (2X) 5 μL Deionized Water to 5 μL Total Volume 10 μL Do not heat samples for native electrophoresis.
Prepare 1X Buffer
Add 100 mL 10X Tris-Glycine Native Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine Native Running Buffer.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Load Buffer
Fill the Upper Buffer Chamber with 200 mL and the Lower Buffer Chamber with 600 mL of 1X Tris-Glycine Native Running Buffer.
Run
Voltage: Run Time: Expected Current:
Prepare Samples
Denaturing Samples: Add 50 mL 20X NuPAGE Tris-Acetate SDS Running Buffer to 950 mL deionized water. Native Samples: Add 100 mL 10X TrisGlycine Native Running Buffer to 900 mL deionized water.
Reagent Reduced Sample Sample x μL Tris-Glycine SDS Sample Buffer (2X) 5 μL NuPAGE Reducing Agent (10X) 1 μL Deionized Water to 4 μL Total Volume 10 μL
Non-reduced Sample x μL 5 μL -to 5 μL 10 μL
Heat samples at 85˚C for 2 minutes.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Prepare 1X Buffer
Load Buffer
Fill the Upper (200 mL) and Lower (600 mL) Buffer Chambers with the appropriate 1X Running Buffer. For reduced samples, use 200 mL 1X Running Buffer with 500 μL NuPAGE Antioxidant in the Upper Buffer Chamber.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Load Buffer
Fill the Upper Buffer Chamber with 200 mL and the Lower Buffer Chamber with 600 mL of 1X Tris-Glycine SDS Running Buffer.
Conditions
Run
Voltage: Run Time: Expected Current:
Blot Gel
Run Conditions
Voltage: Run Time: Expected Current:
150 V constant 1 hour (Denaturing gel), 2–3 hours (Native gel) 40–55 mA/gel (start); 25–40 mA/gel (end) for denaturing gel 18 mA/gel (start); 7 mA/gel (end) for native gel
Conditions
Add 100 mL 10X Novex Tris-Glycine SDS Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine SDS Running Buffer.
125 V constant 90 minutes (dependent on gel percentage) 30–40 mA/gel (start); 8–12 mA/gel (end)
125 V constant 1–12 hours 6–12 mA/gel (start); 3–6 mA/gel (end)
For blotting denaturing and native gels, use 1X Tris-Glycine Transfer Buffer with 20% methanol. Perform blotting at 25 V constant for 1–2 hours using the XCell II Blot Module. The expected start current is 100 mA.
Intended Use: For research use only. Not for human or animal therapeutic or diagnostic use.
Appendix
78
Protein gel electrophoresis technical handbook
Appendix
79
Protocol quick reference QUICK REFERENCE
QUICK REFERENCE
Tris-Glycine Midi Gels
Non-denaturing (Native) Electrophoresis
Instructions for electrophoresis using the XCell4 SureLock Midi-Cell are described below.
Prepare Samples
Reagent
Reduced sample
Sample Tris-Glycine SDS Sample Buffer (2X) NuPAGE Reducing Agent (10X) Deionized Water
x µL 5 µL 1 µL to 10 µL final
Non-reduced sample x µL 5 µL — to 10 µL final
Heat samples at 85˚C for 2 minutes.
Prepare 1X Buffer
Add 100 mL 10X Tris-Glycine SDS Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine SDS Running Buffer.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Add Buffer
Fill each Upper Buffer Chamber with 175 mL 1X Tris-Glycine SDS Running Buffer. Fill the Lower Buffer Chamber up to the fill line mark with 1X Tris-Glycine SDS Running Buffer.
Run Conditions
Voltage: Run Time: Expected Current:
Prepare Samples
Reagent Sample Tris-Glycine Native Sample Buffer (2X) Deionized Water
Native Sample x µL 5 µL to 10 µL final
Do not heat native samples.
Prepare 1X Buffer
Add 100 mL 10X Tris-Glycine SDS Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine SDS Running Buffer.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Add Buffer
Fill each Upper Buffer Chamber with 175 mL of 1X Tris-Glycine Native Running Buffer. Fill the Lower Buffer Chamber up to the fill line mark with 1X TrisGlycine Native Running Buffer.
Run Conditions
Voltage: Run Time: Expected Current:
125 V constant 1–12 hours 35–40 mA/gel (start); 15–20 mA/gel
Tricine Gels
Tricine Gels
Instructions are provided below for electrophoresis of Tricine Gels using the XCell SureLock Mini-Cell.
Blotting Conditions
For blotting Tricine gels, use 1X Tris-Glycine Transfer Buffer with 20% methanol. Perform transfer with nitrocellulose or PVDF membranes at 25 V constant for 1–2 hours using the XCell II Blot Module. The expected start current is 100 mA.
Alternate Transfer Buffers
The Tris-Glycine Transfer Buffer interferes with protein sequencing. If you are performing protein sequencing, use 1X NuPAGE Transfer Buffer or 0.5X TBE Transfer Buffer for blotting. The NuPAGE Transfer Buffer protects against modification of the amino acid side chains and is compatible with N-terminal protein sequencing using Edman degradation.
Prepare Samples
Reagent Reduced Sampl e Sam pl e x μL Tricine SDS Sample Buffer (2X) 5 μL NuPAGE Reducing Agent (10X) 1 μL Deionized Water to 4 μL Tot al Vol u m e 10 μ L
Non- r educed Sampl e x μL 5 μL -to 5 μL 10 μ L
Heat samples at 85˚C for 2 minutes.
Prepare 1X Buffer
Add 100 mL 10X Novex Tricine SDS Running Buffer to 900 mL deionized water to prepare 1X Tricine SDS Running Buffer.
Load Sample Load the appropriate concentration of your protein sample on the gel. Load Buffer Fill the Upper Buffer Chamber with 200 mL and the Lower Buffer Chamber
125 V constant 105 min (dependent on gel percentage) 40–50 mA/gel (start); 20–25 mA/gel (end)
with 600 mL of 1X Tricine SDS Running Buffer.
Run Conditions
For Research Use Only. Not for use in diagnostic procedures.
Voltage: Run Time: Expected Current:
125 V constant 90 minutes (dependent on gel percentage) 80 mA/gel (start); 40 mA/gel (end)
For research use only. Not for human or animal therapeutic or diagnostic use.
QUICK REFERENCE
QUICK REFERENCE
Staining Protocol
NativePAGE Bis-Tris Gels ™
Instructions are provided below for electrophoresis of NativePAGE™ Bis-Tris Gels using the XCell SureLock Mini-Cell.
Prepare Samples
Reagent Sample NativePAGE™ Sample Buffer (4X) NativePAGE™ 5% G-250 Additive Deionized Water
Sample with detergent x μL 2.5 μL 0.25–1 μL* to 10 μL
Detergent-free sample x μL 2.5 μL — to 10 μL
Do not heat samples for native gel electrophoresis. *Ensure the final G-250 concentration is ¼th the detergent concentration.
Prepare 1X Running Buffer
1X NativePAGE Anode Buffer: Add 50 mL 20X NativePAGE Running Buffer to 950 mL deionized water. 1X NativePAGE™ Cathode Buffer: Add 50 mL 20X NativePAGE™ Running Buffer and 50 mL 20X NativePAGE™ Cathode Additive to 900 mL deionized water. ™
™
Load Sample Fill wells with 1X NativePAGE™ Cathode Buffer and load samples prior to filling
the cathode chamber. Load an appropriate amount of your sample on the gel.
Add Buffer Run Conditions
A quick staining protocol for NativePAGE Gels using the Coomassie G-250 from the sample additive is described below. The total staining time is ~2–3 hours. Sensitivity is ~60 ng BSA. Step
1 2
Action
Time
Place the gel in 100 mL fixing solution (40% methanol, 10% acetic acid) and microwave on high (950–1100 watts).
45 seconds
Shake the gel on an orbital shaker.
15 minutes
3
Discard fixing solution.
4
Place the gel in 100 mL destain solution (8% acetic acid) and microwave on high (950–1100 watts).
5
Shake the gel on an orbital shaker until the desired background is obtained.
IEF Gels
IEF Gels
Instructions are provided below for electrophoresis of IEF Gels using the XCell SureLock Mini-Cell.
Prepare for 1. Stain and destain the IEF gel. Incubate the IEF gel in 100 mL 20% ethanol 2D SDS/ for 10 minutes. PAGE 2. Cut out the desired lane (strip) from the gel for transfer to a SDS gel.
Prepare Samples
— 45 seconds
Reagent Sample IEF Sample Buffer pH 3–10 or pH 3–7 (2X) Deionized Water
Prepare 1X Buffer
1X IEF Anode Buffer: Add 20 mL 50X IEF Anode Buffer to 980 mL deionized water. Chill to 4°C to 10°C. 1X IEF Cathode Buffer: Add 20 mL IEF Cathode Buffer pH 3–10 (10X) or pH 3–7 (10X) to 180 mL deionized water. Chill to 4°C to 10°C.
Load Sample
Load the appropriate concentration and volume of your protein on the gel.
Add Buffer
Fill the Upper Buffer Chamber with chilled 200 mL 1X IEF Cathode Buffer and the Lower Buffer Chamber with chilled 600 mL 1X IEF Anode Buffer.
Run
Voltage: Expected Current:
—
Conditions
100 V constant for 1 hour 200 V constant for 1 hour 500 V constant for 30 minutes 7 mA/gel (start); 5 mA/gel (end)
Fill the Upper Buffer Chamber with ~200 mL 1X NativePAGE™ Cathode Buffer. Fill the Lower Buffer Chamber with ~550 mL 1X NativePAGE™ Anode Buffer.
Stain Gel
Voltage: Run Time: Expected Current:
For Research Use Only. Not for use in diagnostic procedures.
150 V constant 90–115 min (3–12% gel); 105–120 min (4–16% gel) 12–16 mA/gel (start); 2–4 mA/gel (end)
Sample x μL 5 μL to 10 μL final
Fix the IEF gel in 12% TCA or 12% TCA containing 3.5% sulfosalicylic acid for 30 minutes. Stain the IEF gel with method of choice.
3. Incubate the gel strip in 2 mL 2X SDS sample buffer and 0.5 mL ethanol for 3–5 minutes. Aspirate the sample buffer and rinse the gel strip with 1X SDS Running Buffer. 4. Fill the SDS gel cassette with 1X SDS Running Buffer. 5. Trim the IEF gel strip to a length of 5.8–5.9 cm. 6a. Transfer the gel strip into a 1.0 mm SDS gel by sliding the strip into the 2D-well using a gel loading tip. Avoid trapping air-bubbles between the strip and the SDS gel. Wet a piece of thick filter paper (5.8 cm × 4 cm) in SDS Running Buffer and insert the long edge of the paper into the 2D-well to push the gel strip into with the SDS gel. 6b. If transferring the gel strip into a 1.5 mm SDS gel, wet 2 pieces of thin filter paper (5.8 cm × 4 cm) in 1X SDS Running Buffer. Sandwich the gel strip between the two filter papers along the long edge with the edge of the strip protruding ~0.5 mm beyond the paper. Insert the sandwich into the 2D-well of the SDS gel without trapping air bubbles and push the strip down so it is in with the SDS gel. 7. Insert gel into the mini-cell, fill the buffer chambers with 1X SDS Running Buffer, and perform SDS-PAGE. 8. After the dye front has moved into the stacking gel (~10 minutes), disconnect power, remove the paper, and resume electrophoresis.
For Research Use Only. Not for use in diagnostic procedures.
Appendix
80
Protein gel electrophoresis technical handbook
Appendix
81
Ordering information QUICK REFERENCE
Zymogram Gels
Zymogram Gels
Instructions are provided below for electrophoresis of Zymogram Gels using the XCell SureLock Mini-Cell.
Develop Gel
Prepare Samples
Prepare 1X Buffer
Reagent Sample Sample x μL Tris-Glycine SDS Sample Buffer (2X) 5 μL Deionized Water to 10 μL final Do not heat or reduce samples for Zymogram gels. 1X Tris-Glycine SDS Running Buffer: Add 100 mL 10X Tris-Glycine SDS Running Buffer to 900 mL deionized water.
Load Sample
Load the appropriate concentration and volume of your protein on the gel.
Add Buffer
Fill the Upper Buffer Chamber with 200 mL, and the Lower Buffer Chamber with 600 mL of 1X Tris-Glycine SDS Running Buffer.
Run Conditions
Voltage: Run Time: Expected Current:
125 V constant 90 minutes (dependent on gel percentage) 30–40 mA/gel (start); 8–12 mA/gel (end)
For Research Use Only. Not for use in diagnostic procedures.
Stain Gel
Sensitivity Level
Product
1. Dilute Zymogram Renaturing Buffer (10X) and Zymogram Developing Buffer (10X) 1:9 with deionized water. Prepare 100 mL of each 1X buffer per 1–2 mini-gels. 2. After electrophoresis, incubate the gel in 1X Zymogram Renaturing Buffer for 30 minutes at room temperature with gentle agitation. 3. Decant Zymogram Renaturing Buffer and add 1X Zymogram Developing Buffer to the gel. 4. Equilibrate the gel for 30 minutes at room temperature with gentle agitation. 5. Decant buffer and add fresh 1X Zymogram Developing Buffer to the gel. 6. Incubate the gel at 37°C for at least 4 hours or overnight for maximum sensitivity. Optimize results empirically by varying the sample load or incubation time. Zymogram (Blue Casein) 4–16% gels do not require staining. For non-prestained Zymogram gels, stain the gels with Colloidal Blue Staining Kit or the SimplyBlue Safestain. Areas of protease activity appear as clear bands against a dark background. 10% Zymogram (Gelatin) Gel: 12% Zymogram (Casein) Gel: 4–16% Zymogram (Blue Casein) Gel:
10-6 units of collagenase 7 × 10-4 units of trypsin 1.5 × 10-3 units of trypsin
Quantity
Cat. No.
Product
Quantity
Cat. No.
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 9-well
1 box
NP0307BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.5 mm, 10-well
1 box
NP0315BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.5 mm, 15-well
1 box
NP0316BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 1-well
1 box
NP0344BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 10-well
10 gels
NP0341BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 10-well
2 gels
NP0341PK2
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 12-well
10 gels
NP0342BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 12-well
2 gels
NP0342PK2
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 15-well
1 box
NP0343BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 17-well
1 box
NP0349BOX
1 box
NP0324BOX
NW3010
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 1-well
10 gels
NP0321BOX
NW3012
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 10-well NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 10-well
2 gels
NP0321PK2
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 12-well
10 gels
NP0322BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 12-well
2 gels
NP0322PK2
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 15-well
10 gels
NP0323BOX
Select precast gel Bolt Bis-Tris Plus Gels Bolt 8% Bis-Tris Plus Gels, 10-well
1 box
NW00080BOX
Bolt™ 8% Bis-Tris Plus Gels, 12-well
1 box
NW00082BOX
Bolt™ 8% Bis-Tris Plus Gels, 15-well
1 box
NW00085BOX
Bolt™ 8% Bis-Tris Plus Gels, 17-well
1 box
NW00087BOX
Bolt™ 10% Bis-Tris Plus Gels, 10-well
1 box
NW00100BOX
Bolt 10% Bis-Tris Plus Gels, 12-well
1 box
NW00102BOX
Bolt™ 10% Bis-Tris Plus Gels, 15-well
1 box
NW00105BOX
Bolt 10% Bis-Tris Plus Gels, 17-well
1 box
NW00107BOX
Bolt 12% Bis-Tris Plus Gels, 10-well
1 box
NW00120BOX
Bolt™ 12% Bis-Tris Plus Gels, 12-well
1 box
NW00122BOX
Bolt 12% Bis-Tris Plus Gels, 15-well
1 box
NW00125BOX
Bolt™ 12% Bis-Tris Plus Gels, 17-well
1 box
NW00127BOX
Bolt 4–12% Bis-Tris Plus Gels, 10-well
1 box
NW04120BOX
Bolt 4–12% Bis-Tris Plus Gels, 12-well
1 box
NW04122BOX
Bolt™ 4–12% Bis-Tris Plus Gels, 15-well
1 box
NW04125BOX
Bolt 4–12% Bis-Tris Plus Gels, 17-well
1 box
NW04127BOX
Bolt™ Empty Mini Gel Cassettes
20 cassettes NW2010
Bolt Empty Mini Gel Cassette Combs, 10-well
20 combs
Bolt™ Empty Mini Gel Cassette Combs, 12-well
20 combs
Bolt Welcome Pack B, 4–12%, 15-well
1 kit**
NW0412B
Bolt™ Welcome Pack A, 4–12%, 10-well
1 kit**
NW0412A
™
™
™
™
™
™
™
™
™
™
One box contains 10 gels. ** B olt Welcome Pack kit includes Mini Gel Tank, 2 boxes of Bolt 4–12% Gels (10-well/15-well), Bolt MES Running Buffer (20X), Bolt LDS Sample Buffer (4X), Bolt Sample Reducing Agent (10X) and SeeBlue™ Plus2 Prestained Standard
NuPAGE Bis-Tris Mini Gels (8 cm x 8 cm) NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 1-well
1 box
NP0304BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 15-well
2 gels
NP0323PK2
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 10-well
10 gels
NP0301BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 17-well
10 gels
NP0329BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 10-well
2 gels
NP0301PK2
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 17-well
2 gels
NP0329PK2
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 12-well
10 gels
NP0302BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 9-well
1 box
NP0327BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 12-well
2 gels
NP0302PK2
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.5 mm, 10-well
10 gels
NP0335BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 15-well
1 box
NP0303BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.5 mm, 10-well
2 gels
NP0335PK2
Appendix
82
Protein gel electrophoresis technical handbook
Appendix
83
Ordering information Product
Quantity
Cat. No.
Product
Quantity
Cat. No.
Product
Quantity
Cat. No.
Product
Quantity
Cat. No.
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.5 mm, 15-well
10 gels
NP0336BOX
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 10-well
2 gels
EA0375PK2
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6078BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6505BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.5 mm, 15-well
2 gels
NP0336PK2
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 12-well
10 gels
EA03752BOX
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC60785BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC65052BOX
2 gels
EA03752PK2
Novex™ 10–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6135BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC65055BOX
WG1201BOX
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 12-well
1 box
EA03755BOX
Novex™ 10–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC61352BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6508BOX
WG1201A
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 15-well
1 box
EA0378BOX
Novex™ 10–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC61355BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC65085BOX
WG1202BOX
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.5 mm, 10-well
1 box
EA03785BOX
Novex™ 10–20% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC61385BOX
Novex™ 4% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6055BOX
WG1202A
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.5 mm, 15-well
1 box
EA0355BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.0 mm, 1-well
1 box
EC6001BOX
Novex™ 4% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60552BOX
WG1203BOX
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.0 mm, 10-well
1 box
EA03552BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6005BOX
Novex™ 4% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6058BOX
WG1203A
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.0 mm, 12-well
1 box
EA03555BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60052BOX
Novex™ 4% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC60585BOX
WG1401BOX
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.0 mm, 15-well
1 box
EA0358BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC60055BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6035BOX
WG1401A
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.5 mm, 10-well
1 box
EA03585BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6008BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60352BOX
WG1402BOX
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.5 mm, 15-well
1 box
EC60085BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC60355BOX
WG1601BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC6485BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6038BOX
WG1601A
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC64852BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC60385BOX
WG1602BOX
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC64855BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 1-well
1 box
EC6021BOX
WG1602A
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC6488BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6025BOX
WG1603BOX
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC64885BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60252BOX
WG1603A
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well Novex™ 16% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6495BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC60255BOX
NuPAGE Bis-Tris Midi Gels (8 cm x 13 cm) NuPAGE Novex 10% Bis-Tris Midi Protein Gels, 12+2-well
1 box
NuPAGE Novex 10% Bis-Tris Midi Protein Gels, 12+2-well, w/adapters
1 box
NuPAGE™ Novex™ 10% Bis-Tris Midi Protein Gels, 20-well
1 box
NuPAGE Novex 10% Bis-Tris Midi Protein Gels, 20-well, w/adapters
1 box
NuPAGE Novex 10% Bis-Tris Midi Protein Gels, 26-well
1 box
NuPAGE™ Novex™ 10% Bis-Tris Midi Protein Gels, 26-well, w/adapters
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 12+2-well
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 12+2-well, w/adapters
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 20-well
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 20-well, w/adapters
1 box
WG1402A
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 12+2W
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 26-well
1 box
WG1403BOX
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 12+2W, w/adapters
1 box
™
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 26-well, w/adapters
1 box
WG1403A
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 20W
1 box
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 12+2-well
1 box
WG1001BOX
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 20W, w/adapters
1 box
™
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 12+2-well, w/adapters
1 box
WG1001A
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 26W
1 box
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 20-well
1 box
WG1002BOX
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 26W, w/adapters
1 box
NuPAGE™ Novex™ 8% Bis-Tris Midi Protein Gels, 20-well, w/adapters
1 box
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 26-well
1 box
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 26-well, w/adapters
1 box
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
NuPAGE Tris-Acetate Midi Gels (8 cm x 8 cm)
10 gels
™
™
™
™
™
™
™
™
™
™
™
WG1002A
Novex Tris-Glycine Mini Gels (8 cm x 8 cm) 1 box
EC6075BOX
Novex™ 16% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC64952BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 9-well
1 box
EC60249BOX
WG1003BOX
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
3 boxes
EC6075BX30
Novex™ 16% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC64955BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6028BOX
WG1003A
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well - Value Pack Novex™ 10% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60752BOX
Novex™ 16% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6498BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC60285BOX
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC60755BOX
Novex™ 16% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC64985BOX
Novex™ 6% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6065BOX
NuPAGE Tris-Acetate Mini Gels (8 cm x 8 cm) NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 10-well
™
EA0375BOX
Appendix
84
Protein gel electrophoresis technical handbook
Appendix
85
Ordering information Product
Quantity
Cat. No.
Product
Quantity
Cat. No.
Product
Novex™ 6% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60652BOX
Novex™ 4–20% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
WT4201A
Novex Tricine Gels 1 box
EC6675BOX
Pierce™ SDS-PAGE Sample Prep Kit
1 box
Novex 4–20% Tris-Glycine Midi Protein Gels, 20-well
1 box
WT4202BOX
50 reactions
89888
Novex 6% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
Novex 10% Tricine Protein Gels, 1.0 mm, 10-well
1 box
EC66752BOX
Bolt Transfer Buffer (20X)
125 mL
BT0006
1 box
WT4202A
Bolt Transfer Buffer (20X)
1L
BT00061
WT0101BOX
Novex 4–20% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
Novex™ 10% Tricine Protein Gels, 1.0 mm, 12-well
1 box
EC6695BOX
4X Bolt LDS Sample Buffer
10 mL
B0007
1 box
WT4203BOX
WT0101A
Novex™ 4–20% Tris-Glycine Midi Protein Gels, 26-well
Novex™ 16% Tricine Protein Gels, 1.0 mm, 10-well
1 box
EC66952BOX
20X Bolt MES SDS Running Buffer
500 mL
B0002
1 box
WT4203A
WT0102BOX
Novex™ 4–20% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
Novex™ 16% Tricine Protein Gels, 1.0 mm, 12-well
1 box
WT0102A
Novex™ 8% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
WT0103BOX
Novex™ 8% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
WT0103A
Novex™ 8% Tris-Glycine Midi Protein Gels, 20-well
1 box
WT0121BOX
Novex™ 8% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
WT0083BOX
Novex™ pH 3–7 IEF Protein Gels, 1.0 mm, 12-well
5 gels⁄box
WT0121A
Novex™ 8% Tris-Glycine Midi Protein Gels, 26-well
1 box
WT0083A
WT0122BOX
Novex™ 8% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
Novex™ pH 3–7 IEF Protein Gels, 1.0 mm, 10-well
1 box
WT8161BOX
WT0122A
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 12+2-well
Novex™ pH 3–10 IEF Protein Gels, 1.0 mm, 10-well
1 box
WT8161A
Novex Zymogram Gels
WT0123BOX
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
WT0123A
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 20-well
1 box
WT4121BOX
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
WT4121A
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 26-well Novex™ 8–16% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
1 box
™
EC60655BOX
Novex Tris-Glycine Midi Gels (8 cm x 13 cm) Novex™ 10% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
Novex™ 10% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
Novex™ 10% Tris-Glycine Midi Protein Gels, 20-well
1 box
Novex 10% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
Novex™ 10% Tris-Glycine Midi Protein Gels, 26-well
1 box
Novex™ 10% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 20-well
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 26-well
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
1 box
Novex 4–12% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
Novex 4–12% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
™
™
™
™
™
™
™
™
™
™
™
™
WT4122BOX
Novex™ 4–12% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
WT4122A
NativePAGE™ Novex™ 3–12% Bis-Tris Protein Gels, 1.0 mm, 10-well
1 box
Novex™ 4–12% Tris-Glycine Midi Protein Gels, 26-well
1 box
WT4123BOX
NativePAGE™ Novex™ 3–12% Bis-Tris Protein Gels, 1.0 mm, 15-well
1 box
Novex 4–12% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
1 box
NativePAGE Novex 4–16% Bis-Tris Protein Gels, 1.0 mm, 10-well
1 box
Novex™ 4–20% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
NativePAGE™ Novex™ 4–16% Bis-Tris Protein Gels, 1.0 mm, 15-well
1 box
WT4123A WT4201BOX
Quantity
Cat. No.
EC66955BOX
20X Bolt MES SDS Running Buffer
5L
B0002-02
20X Bolt MOPS SDS Running Buffer
500 mL
B0001
20X Bolt MOPS SDS Running Buffer
5L
B0001-02
1 box
WT0081BOX
Novex 10–20% Tricine Protein Gels, 1.0 mm, 10-well
1 box
EC6625BOX
Bolt Antioxidant
15 mL
BT0005
WT0081A
LA0041
1 box
EC66252BOX
NuPAGE Tris-Acetate SDS Running Buffer (20X)
500 mL
WT0082BOX
Novex™ 10–20% Tricine Protein Gels, 1.0 mm, 12-well
NP0001
1 box
EC66255BOX
NuPAGE™ MOPS SDS Running Buffer (20X)
500 mL
WT0082A
Novex™ 10–20% Tricine Protein Gels, 1.0 mm, 15-well
NuPAGE™ MOPS SDS Running Buffer (20X)
5L
NP000102
EC66452BOX
NuPAGE™ MES SDS Running Buffer (20X)
5L
NP000202
5 gels⁄box
EC6645BOX
NuPAGE™ MES SDS Running Buffer (20X)
500 mL
NP0002
5 gels⁄box
EC6655BOX
Novex Tris-Glycine SDS Running Buffer (10X)
4x1L
LC26754 LC2675
1 box
EC64052BOX
Novex Tris-Glycine SDS Running Buffer (10X)
500 mL
WT8162BOX
Novex™ 12% Zymogram (Casein) Protein Gels, 1.0 mm, 12-well
LC26755
1 box
EC6415BOX
Novex Tris-Glycine SDS Running Buffer (10X)
5L
WT8162A
Novex™ 4–16% Zymogram (Blue Casein) Protein Gels, 1.0 mm, 10-well
LC1675
1 box
EC6405BOX
Novex™ Tricine SDS Running Buffer (10X)
500 mL
WT8163BOX
Novex™ 12% Zymogram (Casein) Protein Gels, 1.0 mm, 10-well
NuPAGE™ LDS Sample Buffer (4X)
10 mL
NP0007
1 box
EC61755BOX
WT8163A
Novex™ 10% Zymogram (Gelatin) Protein Gels, 1.0 mm, 15-well
Novex Tricine SDS Sample Buffer (2X)
20 mL
LC1676
20 mL
LC2676
™
Novex™ 10% Zymogram (Gelatin) Protein Gels, 1.0 mm, 12-well
1 box
EC61752BOX
Novex Tris-Glycine SDS Sample Buffer (2X)
BN1001BOX
Novex™ 10% Zymogram (Gelatin) Protein Gels, 1.0 mm, 10-well
1 box
EC6175BOX
Novex™ Tris-Glycine Transfer Buffer (25X)
500 mL
LC3675
BN1003BOX
E-PAGE™ High Throughput Gel System
NuPAGE™ Transfer Buffer (20X)
125 mL
NP0006
E-PAGE™ 8% Protein Gels, 48-well
EP04808
NuPAGE Transfer Buffer (20X)
1L
NP00061
EH03
NuPAGE Antioxidant
15 mL
NP0005
EPBUF01
Novex Tris-Glycine SDS Buffer Kit
1 kit
LC2677
NuPAGE MOPS SDS Buffer Kit (for Bis-Tris Gels)
1 kit
NP0050
NuPAGE™ MES SDS Buffer Kit (for Bis-Tris Gels)
1 kit
NP0060
NativePAGE Gels
™
Product
Prepare samples and select buffers: SDS-PAGE
Novex IEF Gels
1 box
™
Cat. No.
Novex™ 16% Tricine Protein Gels, 1.0 mm, 15-well ™
Novex™ 4–12% Tris-Glycine Midi Protein Gels, 20-well
™
Quantity
BN1002BOX BN1004BOX
E-Holder™ Platform E-PAGE™ Loading Buffer 1
8 gels 2 units 4.5 mL
E-PAGE™ 6% Protein Gels, 96-well
8 gels
EP09606
Daughter E-Base™ Device
1 unit
EBD03
Mother E-Base Device
1 unit
EBM03
™
™
™
™
™
Appendix
86
Protein gel electrophoresis technical handbook
Appendix
87
Ordering information Product
Quantity
Cat. No.
Product
NuPAGE™ Tris-Acetate SDS Buffer Kit (for Tris-Acetate gels), Contains 1 ea. LA0041, NP0004, NP0005, NP0007
1 kit
LA0050
Select Protein Standards: Unstained
Novex™ Tricine SDS Buffer Kit, includes LC1676 & LC1675
1 kit
Pierce LDS Sample Buffer, NonReducing (4X) Pierce Lane Marker Non-Reducing Sample Buffer
5 mL 5 mL
LC1677 84788 39001
Pierce 10X Tris-Glycine SDS Buffer
1L
28362
BupH Tris-Glycine-SDS Buffer Packs
40 packs
28378
™
Native Electrophoresis Novex Tris-Glycine Native Running Buffer (10X)
500 mL
LC2672
Novex Tris-Glycine Native Sample Buffer (2X)
20 mL
LC2673
NativePAGE™ Running Buffer (20X)
1L
BN2001
NativePAGE™ Running Buffer Kit
1 kit
BN2007
NativePAGE™ Cathode Buffer Additive (20X)
250 mL
BN2002
NativePAGE™ Sample Buffer (4X)
Quantity
Cat. No.
PageRuler™ Unstained Low Range Protein Ladder
2 x 250 µL
26632
PageRuler™ Unstained Protein Ladder
2 x 250 µL
26614
NativeMark Unstained Protein Standard
5 x 50 µL
LC0725
Prestained
BN2003
Mini Gel Tank
1 unit
A25977
PageBlue Protein Staining Solution
1L
24620
XCell SureLock Mini-Cell
1 unit
EI0001
SimplyBlue SafeStain
1L
LC6060
XCell4 SureLock™ Midi-Cell
1 each
WR0100
SimplyBlue SafeStain
3.5 L
LC6065
PowerEase™ 90W Power Supply (115 VAC)
1 each
PS0090
Imperial Protein Stain
1L
24615
Imperial Protein Stain
3x1L
24617
PowerEase™ 90W Power Supply (230 VAC)
1 each
PS0091
Pierce™ Silver Stain Kit
1L
24612
SilverXpress™ Silver Staining Kit
1 kit*
LC6100
Pierce™ Silver Stain for Mass Spectrometry
1L
24600
SYPRO™ Orange Protein Gel Stain
500 µL
S-6650
SYPRO™ Orange Protein Gel Stain
10 x 50 µL
S-6651
™
™
2 x 250 µL
26619
PowerEase™ 300W Power Supply (115 VAC)
1 each
PS0300
PageRuler™ Plus Prestained Protein Ladder
10 x 250 µL
26620
PowerEase™ 300W Power Supply (230 VAC)
1 each
PS0301
Spectra™ Multicolor Broad Range Protein Ladder
2 x 250 µL
26634
Spectra™ Multicolor Broad Range Protein Ladder
10 x 250 µL
26623
HiMark Prestained Protein Standard
250 µL
LC5699
SYPRO™ Red Protein Gel Stain
500 µL
S-6653
Spectra Multicolor High Range Protein Ladder
2 x 250 µL
26625
SYPRO™ Red Protein Gel Stain
10 x 50 µL
S-6654
SYPRO Ruby Protein Gel Stain
1L
S-12000
SYPRO Ruby Protein Gel Stain
200 mL
S-12001
SYPRO Ruby Protein Gel Stain
5L
S-21900
Pro-Q Emerald 488 Glycoprotein Gel Stain Kit
1 kit
P-21875
Pro-Q™ Diamond Phosophoprotein Gel Stain Kit
1L
P-33300
Pro-Q™ Diamond Phosophoprotein Gel Stain Kit
200 mL
P-33301
Pro-Q™ Diamond Phosophoprotein Gel Stain Kit
5L
P-33302
Pierce Power Stainer
1 unit
22833
Pierce Power Stainer Welcome Pack
1 unit
22833SPCL*
™
™
Silver stains
*1 kit contains sufficient reagents to stain 25 mini gels
Fluorescent and specialty stains
™
™
MagicMark™ XP Western Protein Standard
250 µL
LC5602
DDM (n-dodecyl β-D-maltoside) (10%)
1 mL
BN2005
Specialty
1 mL
BN2006
PageRuler Prestained NIR Protein Ladder
2 x 250 µL
500 mL
LC2671
BenchMark™ Fluorescent Protein Standard
125 µL
LC2670
BenchMark His-tagged Protein Standard
125 µL
LC5606
IEF Marker 3–10
500 µL
39212-01
™
™
IEF
Coomassie stains
PageRuler™ Plus Prestained Protein Ladder
BN2008
500 mL
Electrophoresis chamber systems and power supplies
26617
1 kit
Novex™ Zymogram Renaturing Buffer (10X)
50 µL
LC5603
26635 LC5928
™
™
Pierce Power Stainer
™
Novex™ IEF Anode Buffer (50X)
100 mL
LC5300
Novex™ IEF Cathode Buffer pH 3-10 (10X) 125 mL
LC5310
Novex™ IEF Cathode Buffer pH 3-7 (10X)
125 mL
LC5370
Novex pH 3-10 IEF Buffer Kit, Includes LC5300, LC5310, LC5311
1 kit
LC5317
Novex™ pH 3-7 IEF Buffer Kit, Includes LC5300, LC5370, LC5371
1 kit
LC5377
Novex™ IEF Sample Buffer pH 3-10 (2X)
25 mL
LC5311
Novex IEF Sample Buffer pH 3-7 (2X)
25 mL
LC5371
™
™
Cat. No.
10 x 250 µL
NativePAGE Sample Prep Kit
Novex™ Zymogram Developing Buffer (10X)
Quantity
PageRuler™ Prestained Protein Ladder
MagicMark™ XP Western Protein Standard
Zymography
Product
26616
0.5 mL
Digitonin (5%)
Cat. No.
2 x 250 µL
NativePAGE™ 5% G-250 Sample Additive ™
BN2004
Quantity
PageRuler™ Prestained Protein Ladder
Western 10 mL
Product
*Welcome pack includes Pierce Power Station, Pierce Power Stain Cassette, Western Blot Roller, Power Cord with C/13 Connector, Quick Start Guide, Pierce Mini Gel Power Staining Kit
Appendix Appendix
Crossword puzzle challenge To participate in the crossword puzzle challenge, Separate Crossword
go to thermofisher.com/pagecrossword Complete the crossword below 1
2
3 4 5
6
7 8
9
10 11
12
13
14
15
Across
Created on TheTeachersCorner.net Crossword Maker
Xcell4SureLock
DownHermannSchagger UlrichLaemmli ArneTiselius
SheldonEngelhorn
5. Can you name one of the scientists who developed 1. Which tank is compatible with >180 mini gels? pg. 52 BisTris MagicMark PowerStainer Bolt minimizes Minigeltank HarrySvennsonRilbe blue native polyacrylamide gel electrophoresis? 2. Which gel chemistry protein degradation? pg. 9 pg. 17Unstained 6. Which tank is compatible with midigels? pg. 56 3. Who first published SDS-PAGE as a method for the Prestained PowerEase NuPAGE 9. Which ladder can be used for accurate molecular analysis of cleavage of structural proteins in weight estimation directly on western blots? pg. 46 bacteriophage? pg. 7 10. Can you name one of the scientists who filed a 4. Can you name one of the scientists who first patent for the netural-pH Bis-Tris system in 1996? pg. 11 described the theory of separation of amphoteric 12. Which gel has a unique wedge well design that proteins along a pH gradient in the 1960s? pg. 21 allows you to load 2x the sample volume? pg. 10 7. What power supply is available for use with the Mini 13. Which protein ladder would you use for Gel Tank? pg. 58 approximate determination of molecular weight? pg. 35 8. What is a fast alternative to traditional Coomassie 15. Who won the Nobel Prize for analysis of serum staining? pg. 72 proteins by electrophoresis in 1948? pg. 5 11. Which ladder would you use for precise determination of molecular weight? pg. 35 14. Which type of gel has been referenced in >20,000 publications? pg. 12
Learn more at thermofisher.com/invitrogen For Research Use Only. Not for use in diagnostic procedures. © 2015 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. Polaroid is a trademark of Polaroid Corporation. Triton is a trademark of Union Carbide Corporaton. CO015381 0715
Protein gel electrophoresis technical handbook separate transfer detect
2
Select precast gel
Comprehensive solutions designed to drive your success
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Post stain
Contents Electrophoresis overview
4
Select precast gel
Protein gel electrophoresis is a simple way to separate proteins prior to downstream detection or analysis, and is a critical step in most workflows that isolate, identify, and characterize proteins. We offer a complete array of products to rapid, reliable protein electrophoresis for a variety of applications, whether it is the first or last step in your workflow. Our portfolio of high-quality protein electrophoresis products unites gels, stains, molecular weight markers, running buffers, and blotting products for your experiments.
8 10
Gel selection guide Gels
Prepare samples and select buffers Sample prep kits 26 Buffers and reagents 28 Buffers and reagents selection guide 29
Select the standard Protein ladders 34 Protein standards selection guide 36
Choose the electrophoresis chamber system and power supply Electrophoresis chamber systems 50 Electrophoresis chamber system selection guide 51 Power supplies 58
Run the gel Gel run conditions 59 Troubleshooting tips 60
Stain the gel Protein stains 62 Protein stains selection guides 63, 67, 69, 70 Electrophoretic staining technology 71
Post stain Transfer and detection 74
Appendix Protocol quick reference 76 Ordering information 81
For a complete listing of all available products and more, visit thermofisher.com/separate
For ordering information refer to page XX. For quick reference on the protocol please refer to page XX.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
3
4
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Post stain
Electrophoresis matrix Electrophoresis is defined as the Two types of matrices are commonly used in transport of charged molecules electrophoresis—polyacrylamide and agarose. The matrices act as porous media and behave like a molecular sieve. through a solvent by an electric Separation of molecules is dependent upon the gel pore size of the matrix used. Agarose has a large pore size and is ideal field. Electrophoresis is a simple, for separating macromolecules such as nucleic acids and protein complexes. Polyacrylamide has a smaller pore size and is ideal for rapid, and sensitive analytical separating most proteins and smaller nucleic acids. tool for separating proteins and Polyacrylamide gel electrophoresis nucleic acids. Any charged ion or (PAGE) molecule will migrate when placed Polyacrylamide gels are generated by the polymerization of acrylamide monomers. These monomers are crosslinked into in an electric field. Most biological long chains by the addition of bifunctional compounds such as (bis), which react with the free molecules carry a net charge at any N,N,-methylenebisacrylamide functional groups of the chain termini. The concentration of acrylamide and bisacrylamide determines the pore size of the gel. pH other than at their isoelectric The higher the acrylamide concentration, the smaller the pore size, resulting in resolution of lower molecular weight molecules and point and will migrate at a rate vice versa. proportional to their charge density. PAGE allows one to separate proteins for different applications
The mobility of a biological molecule through an electric field will depend on the following factors:
based on: • The acrylamide matrix • Buffer systems
Electrophoresis conditions
Linear vs. gradient gels
The separation of molecules is dependent on the electrophoresis conditions. Electrophoresis can be performed under the following conditions:
Gels that have a single acrylamide percentage are referred to as linear gels, and those with a range are referred to as gradient gels. The advantage of using a gradient gel is that it allows the separation of a broader range of proteins than a linear gel.
Continuous vs. discontinuous gels Researchers occasionally refer to gels as continuous or discontinuous. A continuous gel is a gel that has been formed from a single acrylamide solution in the entire gel cassette. A discontinuous gel is formed from two acrylamide solutions, a small, low-percentage stacking gel where the protein wells reside, and a larger portion of gel that separates the proteins. In the traditional Tris-glycine protein gel system, the proteins are stacked in the stacking gel between the highly mobile leading chloride ions (in the gel buffer) and the slower, trailing glycine ions (in the running buffer). The reason for using the stacking gel is to improve the resolution of the bands in the gel. These stacked protein bands undergo sieving once they reach the separating gel.
Mini vs. midi protein gels Commercial gels are available in two size formats, minigels and midigels. Both gels have similar run lengths, but midigels are wider than minigels, allowing midigels to have more wells or larger wells. The additional wells in the midigels permit more samples or large sample volumes to be loaded onto one gel.
Denaturing conditions Electrophoresis is performed under denaturing conditions using an anionic detergent such as sodium dodecylsulfate (SDS). SDS denatures and unfolds the protein by wrapping around the hydrophobic portions. SDS binds at a ratio of ~1.4 g SDS per gram of protein. The resultant SDS-protein complexes are highly negatively charged and are resolved in the gel based on their size.
Nondenaturing (native) conditions Electrophoresis is performed under nondenaturing (native) conditions using buffer systems that maintain the native protein conformation, subunit interaction, and biological activity. During native electrophoresis, proteins are separated based on their charge to mass ratios.
Reducing conditions Electrophoresis is performed under reducing conditions using reducing agents such as dithiothreitol (DTT), β-mercaptoethanol (β-ME) or tris(2-carboxyethyl)phosphine (TCEP). The reducing agents completely unfold the denatured proteins into their subunits by cleaving the disulfide bonds between cysteine residues.
Buffer systems
• Electrophoresis conditions
Electrophoresis is performed using continuous or discontinuous buffer systems. A continuous buffer system utilizes only one buffer in the gel and running buffer. A discontinuous buffer system utilizes a different gel buffer and running buffer1. This system may also use two gel layers of different pore sizes and different buffer composition (the stacking and separating gel). Electrophoresis using a discontinuous buffer system results in concentration of the sample and higher resolution.
• Field strength • Net charge on the molecule • Size and shape of the molecule • Ionic strength
Did you know? Arne Tiselius won the Nobel Prize in Chemistry for electrophoretic analysis of serum proteins in 1948.
Reference
• Properties of the matrix through which the molecules migrate (e.g., viscosity, pore size)
The acrylamide matrix
1. Ornstein L (1964) Disc electrophoresis. 1. Background and theory. Ann N Y Acad Sci 121:321-349.
Mini Gel Tank
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
5
6
Select precast gel
Comparison of discontinuous buffer systems SDS-PAGE utilizes a discontinuous buffer system to concentrate or “stack” samples into a very sharp zone in the stacking gel at the beginning of the run. In a discontinuous buffer system, the primary anion in the gel is different (or discontinuous) from the primary anion in the running buffer. Both the Invitrogen™ NuPAGE™ systems (Bis-Tris and Tris-acetate gels) and the Laemmli (Tris-glycine) system are examples of discontinuous buffer systems and work in a similar fashion. However, the NuPAGE system operates at a lower pH as a result of the proprietary ions that are in the system. In a Tris-glycine system (Figure 1), three ions are primarily involved: •C hloride (–), supplied by the gel buffer, serves as the leading ion because it has the highest attraction to the anode relative to other anions in the system. • Glycine (–), the primary anion provided by the running buffer, serves as the trailing ion, because it is only partially negatively charged and remains behind the more highly charged chloride ions in a charged environment. • Tris base (+), is a common ion present in both the gel and the running buffers. During electrophoresis, the gel and buffer ions in the Tris-glycine system form an operating pH of 9.5 in the separating region of the gel. In the case of the Bis-Tris system (Figure 2), three ions are primarily involved: • Chloride (–) supplied by the gel buffer, serves as the fast-moving leading ion. • MES or MOPS (–) (depending on the running buffer choice) serves as the trailing ion. ∙ MES: 2-(N-morpholino) ethane sulfonic acid ∙ MOPS: 3-(N-morpholino) propane sulfonic acid • Bis-Tris (+) acts as the common ion present in the gel while Tris (+) is provided by the running buffer.
With the Tris-acetate system (Figure 3), three ions are primarily involved: • Acetate (–), the leading ion from the gel buffer • Tricine (–), the trailing ion from the running buffer • Tris (+), the common ion (in both gel and running buffer) This system also operates at a significantly lower pH than the Trisglycine system, resulting in less gel-induced protein modifications. The diagrams below (Figures 1, 2, and 3) summarize the migration differences in the stacking gel of each system.
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Post stain
7
Select precast gel High-performance precast protein gels If you are doing standard one-dimensional protein electrophoresis, we have a broad range of solutions to fit your research needs. Our selection of precast gels consists of several different chemistries, well formats, and gel sizes, so you can get the protein separation you need for accurate downstream results. Bolt Bis-Tris Plus gel.
Learn more at thermofisher.com/proteingels GLYCINE (Trailing Ion)
PROTEIN/SDS COMPLEX (Stacked Proteins)
CHLORIDE (Leading Ion)
PROGRESSION OF RUN
Figure 1. The Tris-glycine gel system. • Gel buffer ions are Tris and chloride (pH 8.7) • Running buffer ions are Tris, glycine, and SDS (pH 8.3) • Gel operating pH is 9.5
Common Ion is Tris, present in the gel and running buffers
MES or MOPS (Trailing Ion)
PROTEIN/SDS COMPLEX (Stacked Proteins)
CHLORIDE (Leading Ion)
Figure 2. The Bis-Tris gel system. • Gel buffer ions are Bis-Tris and chloride (pH 6.4) • Running buffer ions are Tris, MES or MOPS, and SDS (pH 7.3) • Gel operating pH is 7.0
Precast gels Popular gel chemistries
Specialty gels
• NuPAGE Bis-Tris gels
• Novex Tricine gels
• NuPAGE Tris-Acetate gels
• NativePAGE gels
• Bolt Bis-Tris Plus gels
• Novex IEF gels
• Novex Tris-Glycine gels
• Novex Zymogram gels • E-PAGE gels
Did you know? Over 45 years ago, Ulrich K. Laemmli first published SDS-PAGE as a method for cleavage analysis of structural proteins in bacteriophage T4.
PROGRESSION OF RUN Common Ion is Bis-tris, present in the gel
Casting your own gels? TRICINE (Trailing Ion)
PROTEIN/SDS COMPLEX (Stacked Proteins)
ACETATE (Leading Ion)
Figure 3. The Tris-acetate gel system. • Gel buffer ions are Tris and acetate (pH 7.0) • Running buffer Ions are Tris, tricine, and SDS (pH 8.3) • Gel operating pH is 8.1
We offer preassembled empty cassettes, buffers, and reagents.
Learn more at thermofisher.com/gelcastingaccessories
PROGRESSION OF RUN Common Ion is Tris, present in the gel and running buffer
The combination of a lower-pH gel buffer (pH 6.4) and running buffer (pH 7.3–7.7) leads to a significantly lower operating pH (pH 7.0) during electrophoresis, resulting in better sample integrity and gel stability.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
8
Prepare samples and select buffers
Select precast gel
Select the standard
Gel selection guide
Choose the electrophoresis chamber system and power supply chamber system and power supply
Run the gel
Stain the gel
Find the right gel for your research needs based on molecular weight, downstream applications, and throughput requirements.
Molecular weight Low molecular weight proteins and peptides (>2.5 kDa)
High molecular weight proteins (<500 kDa)
Novex Tricine gels
NuPAGE Tris-Acetate gels
High-sensitivity western blotting
Broad-range molecular weight separation
Low throughput
Medium or high throughput
Application
E-PAGE 48-well or 96-well gels
Downstream applications requiring high protein integrity (e.g., mass spectrometry)
Large sample volume for high detection sensitivity
Bolt Bis-Tris Plus gels
NuPAGE Bis-Tris gels
NuPAGE Bis-Tris gels
NuPAGE Bis-Tris gels
Bolt Bis-Tris Plus gels
Bolt Bis-Tris Plus gels
Bolt Bis-Tris Plus gels
A
The most widely used gel system for separating a broad range of proteins by SDS-PAGE is the Laemmli system, which uses Tris-glycine gels comprising a stacking gel component that helps focus the proteins into sharp bands at the beginning of the electrophoretic run and the resolving gel component that separates the proteins based on size. This classic system uses a discontinuous buffer system where the pH and ionic strength of the buffer used for running the gel (Tris, pH 8.3) is different from the buffers used in the stacking gel (Tris, pH 6.8) and resolving gel (Tris, pH 8.8). The highly alkaline operating pH of the Laemmli system may cause band distortion, loss of resolution, or artifact bands. The major causes of poor band resolution with the Laemmli system are:
Novex Tris-Glycine gels
• Hydrolysis of polyacrylamide at the high pH of the resolving gel, resulting in a short shelf life of 8 weeks • Chemical alterations such as deamination and alkylation of proteins due to the high pH of the resolving gel
Native separation
Molecular weight 1st dimension 2nd dimension
Isoelectric point
NativePAGE gels
Novex Tris-Glycine gels with native buffers
Novex IEF gels
ZOOM™ IPG strips
NuPAGE Bis-Tris gels, 2D well
Novex Tris-Glycine gels, 2D well
Novex Tris-Glycine gels, 2D well
Novex Tris-Glycine ZOOM™ gels, IPG well
NuPAGE Bis-Tris gels, 2D well
NuPAGE Bis-Tris ZOOM gels, IPG well
Find the right mini gel using our interactive gel selection tool at thermofisher.com/minigelselection
For ordering information refer to pages 81–87.
Protease activity Novex Zymogram gels (casein, blue casein, or gelatin substrates)
9
Choose the right gel chemistry for your research needs Bis-Tris chemistry vs. Tris-glycine chemistry
Denaturing separation
Coomassie dye or silver staining
Protein gel electrophoresis technical handbook
Post stain
1
2
3
4
5
6
7
8
9 10
B
1
2
3
4
5
6
7
8
9
10
Figure 4. Protein separation using (A) a Bolt Bis-Tris Plus gel and (B) another manufacturer’s traditional Tris-glycine gel.
Unlike traditional Tris-glycine gels, NuPAGE and Bolt gels are BisTris HCI–buffered (pH 6.4) and have an operating pH of about 7.0. The neutral operating pH of the Bis-Tris systems provides the following advantages over the Laemmli system: • Longer shelf life of 8–12 months due to improved gel stability • Improved protein stability during electrophoresis at neutral pH enabling sharper band resolution and accurate results (Moos et al. 1998) • Complete reduction of disulfides under mild heating conditions (70°C for 10 minutes) and absence of cleavage of Asp-Pro bonds • Reduced state of the proteins maintained during electrophoresis and blotting of the proteins when using Invitrogen™ NuPAGE™ Antioxidant
• Reoxidation of reduced disulfides from cysteine-containing proteins, as the redox state of the gel is not constant • Cleavage of Asp-Pro bonds of proteins when heated at 100°C in Laemmli sample buffer, pH 5.2
Choosing the right gel percentage In general, the size of the molecule being separated should dictate the acrylamide or agarose percentage you choose. Use a lower percentage gel to resolve larger molecules and a higher percentage gel to resolve smaller ones. The exception to this rule is when performing isoelectric focusing. Refer to the gel migration charts throughout this chapter to find the gel best suited for your application. As a general rule, molecules should migrate through about 70% of the length of the gel for the best resolution. When protein molecular weights are wide ranging, or unknown, gradient gels are usually the best choice.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Choosing a well format and gel thickness We offer most polyacrylamide gels in a choice of nine different well formats (17 well, 15 well, 12 well, 10 well, 9 well, 5 well, 1 well, 2D/preparative well, or IPG well). Two thicknesses (1.0 mm and 1.5 mm) are also available for popular gel types. If loading large sample volumes (>30 μL), a thicker gel (1.5 mm) with fewer wells (e.g., 5 well) or a Bolt gel with its higher-capacity wedge wells is more appropriate. When blotting, that proteins will transfer more easily from a 1.0 mm thick gel than from a 1.5 mm thick gel.
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Bolt Bis-Tris Plus mini gels
Figure 7. Bolt Bis-Tris Plus gel migration chart. Optimal separation range is shown within the gray areas.
Neutral-pH gel system with a unique wedge well design Invitrogen™ Bolt™ Bis-Tris Plus gels are precast polyacrylamide gels designed for optimal separation of a broad molecular weight range of proteins under denaturing conditions during gel electrophoresis (Figure 6 and 7). These gels help deliver consistent performance with a neutral-pH environment to minimize protein degradation. The unique wedge well design (Figure 5) allows loading of up to 2x more sample volume than other precast gels. Bolt gels are ideal for western blot transfer and analysis along with any other technique where protein integrity is crucial. Bolt Bis-Tris Plus gels offer:
Specifications • Shelf life: ~16 months • Average run time: 35 minutes • Separation range: 0.3–260 kDa
• Better protein resolution—gels are 10% longer, allowing detection of more protein bands than standard mini gels • High lot-to-lot consistency—coefficient of variation (CV) of only 2% for Rf values (migration)
Figure 5. The unique wedge well design of Bolt Bis-Tris Plus gels.
“For one of our projects in the lab, we resolve proteins by electrophoresis to determine the accumulation of ubiquitinated proteins following treatment with a proteasome inhibitor. When we resolved the ubiquitinated proteins using the Tris-glycine gels, we observed a smear. However, when we switched to resolving the ubiquitinated proteins using the Bolt Bis-Tris gels, we were delightfully surprised to observe individual protein bands in place of the smear.” —Susan S., University of Pennsylvania, Philadelphia, US
• Polyacrylamide concentrations: fixed 8%, 10%, and 12%; gradient 4–12% • Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per 12-well gel: ~40 μL, or two-thirds of the sample well volume
• Preserved protein integrity—neutral-pH formulation minimizes protein modifications • Superior band quality and band volume— Invitrogen™ Novex™ Bis-Tris Plus chemistry is designed to deliver sharp, straight bands with higher band volume
“The new Bolt system is wonderful. I am still amazed that I can run a PAGE gel in 23 minutes. The entire system is incredibly friendly from the Bolt precast gels with wedged wells for ease of loading to the Mini Gel Tank system. The bands produced from the westerns were sharp and straight. I would and have highly recommended this system to anyone doing protein work.” — Crystal M., Queen’s University, Ontario, Canada
ts acquired w sul ith Re
• High sample volume capacity—wedge well design allows detection of proteins in very dilute samples or measurement of low-abundance proteins
11
Bolt Bis-Tris Plus gels
10
th e
Mini Gel Tan
k
Figure 6. Bolt Bis-Tris Plus gel electrophoresis. Protein standards and samples were loaded at 10 μL sample volumes in a Bolt 4–12% Bis-Tris Plus Gel. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands with consistent migration patterns were observed after staining with Invitrogen™ SimplyBlue™ SafeStain. Images were acquired using a flatbed scanner. Lane 1: Invitrogen™ SeeBlue™ Plus2 Prestained Standard; Lane 2: 10 μg E. coli lysate; Lane 3: Invitrogen™ Mark12™ Unstained Standard (blend of 12 purified proteins); Lane 4: 40 μg HeLa cell lysate; Lane 5: 20 μg HeLa cell lysate; Lane 6: 5 μg BSA; Lane 7: 40 μg Jurkat cell lysate; Lane 8: 5 μg GST fusion protein; Lane 9: Invitrogen™ Novex™ Sharp Unstained Protein Standard; Lane 10: 5 μg β-galactosidase.
Did you know? Timothy Updyke and Sheldon Engelhorn filed a patent for the neutral-pH Bis-Tris gel system in 1996.
Recommended products The Invitrogen™ Bolt™ Welcome Pack + iBlot™ 2 System offers a complete protein separation and western blot solution by combining our Mini Gel Tank, Invitrogen™ Bolt™ gels and buffers, SeeBlue Plus2 Prestained Standard, and Invitrogen™ iBlot™ 2 Gel Transfer Device with transfer stacks.
Thermo Scientific Pierce Power Stainer is recommended for fast Coomassie dye staining of Bolt Bis-Tris Plus Gels.
The Bolt Welcome Pack + iBlot 2 System.
Learn more at thermofisher.com/bolt For ordering information refer to page 81. For quick reference on the protocol please refer to page 76.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
NuPAGE gels
13
NuPAGE gels A.
Revolutionary high-performance gels referenced in >20,000 publications The Invitrogen™ NuPAGE™ SDS-PAGE gel system is a revolutionary high-performance polyacrylamide gel electrophoresis system that simulates the denaturing conditions of the traditional Laemmli system. NuPAGE™ gels use a unique buffer formulation to maintain a neutral operating pH during electrophoresis, which minimizes protein modifications that can result in poor band resolution. Gels are available in two formulations— Invitrogen™ NuPAGE™ Bis-Tris gels are ideal for separating small to midsize proteins while Invitrogen™ NuPAGE™ Tris-Acetate gels are ideal for separating large proteins (Figure 8). A gel migration chart is shown in Figure 9.
NuPAGE Bis-Tris gels (Denaturing separation)
B.
NuPAGE Tris-Acetate gels (Denaturing separation)
C.
NuPAGE Tris-Acetate gels (Native separation)
Specifications • Shelf life: –– NuPAGE Bis-Tris gels: 16 months –– NuPAGE Tris-Acetate gels: 8 months • Average run time: ~35 minutes • Separation range: –– NuPAGE Bis-Tris gels: 1.5–300 kDa –– NuPAGE Tris-Acetate gels: 30–400 kDa
NuPAGE gels are designed for:
• Polyacrylamide concentrations: –– NuPAGE Bis-Tris gels: fixed 8%, 10%, and 12%; gradient 4–12% –– NuPAGE Tris-Acetate gels: fixed 7%; gradient 3–8%
• Superior protein band resolution and stability— neutral-pH environment during electrophoresis minimizes protein modifications
• Gel dimensions: –– Mini: 8 x 8 cm (1 or 1.5 mm thick) –– Midi: 8 x 13 cm (1 mm thick)
• More efficient western blot transfer—neutral pH prevents reoxidation of reduced samples during protein transfer
• Maximum sample volume per 10-well mini gel: 25 µL (1 mm thick); 37 µL (1.5 mm thick)
• Fast sample run times—typically 35–50 minutes • Long product shelf life—stable for 8–16 months
A.
B.
ts acquired w sul ith Re
12
Figure 8. NuPAGE Bis-Tris and Tris-Acetate gel electrophoresis. Protein standards and samples were loaded at 10 μL sample volumes th e k in (A) Invitrogen™ NuPAGE™ 4–12% Bis-Tris and Mini Gel Tan (B) Invitrogen™ NuPAGE™ 3–8% Tris-Acetate gels. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands were observed after staining with SimplyBlue SafeStain. Images were acquired using a flatbed scanner. (A and B) Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 μg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 μg HeLa cell lysate; Lane 5: 20 μg HeLa cell lysate; Lane 6: (A) not used (B) 5 µg BSA; Lane 7: 40 μg Jurkat cell lysate; Lane 8: 5 μg GST fusion protein; Lane 9: Novex Sharp Unstained Protein Standard; Lane 10: 5 μg β-galactosidase.
Learn more at thermofisher.com/nupage For ordering information refer to page 81. For quick reference on the protocol please refer to page 76-77.
Figure 9. Migration patterns achieved in NuPAGE gels. For optimal results, protein bands should migrate within the gray shaded areas. (A) Migration patterns of Invitrogen™ Novex™ Sharp Prestained Protein Standard or Novex Sharp Unstained Protein Standard on NuPAGE Bis-Tris
gels. (B) Migration patterns of HiMark Unstained Protein Standard on NuPAGE Tris-Acetate gels. (C) Migration pattern for Tris-acetate gel native separation is for the Invitrogen™ NativeMark™ Unstained Protein Standard.
Recommended products Invitrogen™ HiMark™ Unstained and Prestained Protein Standards are specifically designed for large protein analysis on NuPAGE Tris-Acetate gels under denaturing conditions. Both standards offer a ready-to-load format and consist of 9 proteins with a size range of 40–500 kDa.
Visualize with Coomassie stain, silver stain, or fluorescent protein stains after electrophoresis (see “Stain the gel”, page 62).
Precast protein gels
PageRuler, PageRuler Plus, and Spectra Prestained Protein Ladders are recommended for use with NuPAGE Bis-Tris gels for easy molecular weight determination.
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Novex Tris-Glycine gels
A.
Laemmli-based precast gels for high efficiency, reproducibility, and performance
Novex Tris-Glycine gels are: • Individually packaged for convenience • Compatible with most protein standards for accurate size determination • Flexible for use with native or denatured protein samples, with specially formulated buffers for each condition
B.
Denaturing separation
Native separation††
Tris-Glycine Gels Large proteins* (116–500 kDa) 0%
Invitrogen™ Novex™ Tris-Glycine gels are based on traditional Laemmli protein electrophoresis with minor modifications for maximum performance in the precast format. These gels provide reproducible separation of a wide range of proteins into wellresolved bands (Figure 10). A gel migration chart is shown in Figure 11.
15
Novex Tris-Glycine gels
Specifications
4%
6%
Small proteins† (3–60 kDa)
Mid-size proteins† (20–250 kDa) 8%
10%
12%
14%
16%
Wide range† (6–200 kDa) 18%
4–12%
8–16%
4–20%
• Shelf life: 1–2 months
10–20%
500 kDa 260kDa kDa 260
500 kDa
10% 260 kDa
• Run time: ~90 minutes
260 kDa 290 kDa
20%
• Separation range: 6–500 kDa
260 kDa
240 kDa
30%
160 kDa
160 kDa
110 kDa
290 kDa
110 kDa 80 kDa
110 kDa
160 kDa
160 kDa 110 kDa
110 kDa
80 kDa
80 kDa
60 kDa
60 kDa
50 kDa 40 kDa
50 kDa
80 kDa 50 kDa
40%
160 kDa
30 kDa
116 kDa
15 kDa 110 kDa 10 kDa
60 kDa 30 kDa
20 kDa
th e
Mini Gel Tan
30 kDa
20 kDa 20 kDa
40 kDa
15 kDa
50 kDa 40 kDa
30 kDa
10 kDa
15 kDa
15 kDa
50 kDa
10 kDa
k
20 kDa
90%
Figure 10. Novex Tris-Glycine gel electrophoresis. Protein standards and samples were loaded at 10 μL sample volumes in 4–20% Tris-Glycine gels. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands were observed after staining with SimplyBlue SafeStain. Images were acquired using a flatbed scanner. Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 μg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 μg HeLa cell lysate; Lane 5: 20 μg HeLa cell lysate; Lane 6: 5 μg BSA; Lane 7: 40 μg Jurkat cell lysate; Lane 8: 5 μg GST fusion protein; Lane 9: Novex Sharp Unstained Protein Standard; Lane 10: 5 μg β-galactosidase.
30 kDa
50 kDa
60 kDa 10 kDa
80%
40 kDa
40 kDa
15 kDa
40 kDa
50 kDa
60 kDa
20 kDa
50 kDa
97 kDa
80 kDa
40 kDa 30 kDa
160 kDa
60 kDa 110 kDa
110 kDa
60%
50 kDa 80 kDa
20 kDa
70%
110 kDa 160 kDa
160 kDa
80 kDa
ts acquired w sul ith Re
60 kDa 260 kDa
60 kDa
• Maximum sample volume per well: 25 μL (1 mm thick); 37 μL (1.5 mm thick)
80 kDa 160 kDa
30 kDa
50 kDa
110 kDa
260 kDa
40 kDa
80 kDa 110 kDa
50%
260 kDa
40 kDa
60 kDa 240 kDa
160 kDa
260 kDa
60 kDa 160 kDa
• Gel dimensions: –– Mini: 8 x 8 cm (1 or 1.5 mm thick) –– Midi: 8 x 13 cm (1 mm thick)
260 kDa
260 kDa 160 kDa
• Polyacrylamide concentrations: –– Fixed concentrations available from 4% to 18% –– Gradient gels with ranges of 4–12%, 4–20%, 8–16%, and 10–20%
14
166 kDa
20 kDa 15 kDa
10 kDa
66 kDa
30 kDa
30 kDa 40 kDa
20 kDa
55 kDa
100%
10 kDa
15 kDa
97 kDa
15 kDa
10 kDa
Figure 11. Migration patterns of protein molecular weight standards in Novex Tris-glycine gels. For optimal results, protein bands should migrate within the gray shaded areas. (A) *Migration patterns of HiMark™ Unstained Protein Standard. † Migration patterns of Novex Sharp PreStained Protein Standard or Novex Sharp Unstained Protein Standard. (B) † † Migration pattern of NativeMARK Unstained Protein Standard.
Recommended products or sample cleanup prior to electrophoresis, we recommend using the F Pierce SDS-PAGE Sample Prep Kit.
Buffers for native proteins: Invitrogen™ Novex™ Tris-Glycine Native Sample Buffer and Novex™ Tris-Glycine Native Running Buffer.
uffers for denatured proteins: Invitrogen™ Novex™ Tris-Glycine SDS B Sample Buffer and Novex™ Tris-Glycine SDS Running Buffer.
PageRuler, PageRuler Plus, and Spectra protein ladders are recommended for molecular weight determination with Novex Tris-Glycine gels.
Learn more at thermofisher.com/trisglycine For ordering information refer to page 82–83. For quick reference on the protocol please refer to page 77-78.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
NativePAGE gels
17
NativePAGE gel
Superior resolution of native proteins and protein complexes The Invitrogen™ NativePAGE™ Bis-Tris gel system is based on the blue native polyacrylamide gel electrophoresis (BN PAGE) technique that uses Coomassie G-250 dye as a charge shift molecule that binds to proteins and confers a negative charge without denaturing the proteins (Figure 12). This technique overcomes the limitations of traditional native electrophoresis by providing a near-neutral operating pH and detergent compatibility. The near-neutral (pH 7.5) environment of the NativePAGE system during electrophoresis results in maximum protein and gel matrix stability, enabling better band resolution than other native gel systems. A gel migration chart is shown in Figure 13. The NativePAGE gel system is designed for:
Specifications • Shelf life: 6 months • Average run time: 90 minutes
Did you know?
• Separation range: 15–10,000 kDa
The blue native polyacrylamide gel electrophoresis technique was developed by Hermann Schagger and Gebhard von Jagow in 1991.
• Polyacrylamide concentrations: gradient 3–12% and 4–16% • Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per 10-well gel: 25 μL
Is there a higher res pic somewhere? I copied and pasted this from source file.
• A wide resolving range—from 15 kDa to over 10 MDa (Figure 12), regardless of isoelectric point • Neutral-pH separation—the native state of protein complexes is better preserved • Superior performance—higher resolution than Tris-glycine native electrophoresis
ts acquired w sul ith Re
dvantages of the NativePAGE gel system over A the Tris-glycine gel system include:
16
th e
Mini Gel Tan
k
Figure 13. NativePAGE gel migration chart. Migration patterns of the NativeMark Unstained Protein Standard on NativePAGE gels are shown.
Figure 12. NativePAGE gel electrophoresis. Two-fold dilution series of protein extracts were run on an Invitrogen™ NativePAGE™ Novex™ 3–12% BisTris Protein Gel using a Mini Gel Tank. Following electrophoresis, the gel was stained with Coomassie dye and imaged using a flatbed scanner. Lanes 1 and 10: blank; Lanes 2 and 6: 5 μL NativeMark Unstained Protein Standard; Lanes 3, 4 and 5: 10, 5, and 2.5 μg spinach chloroplast extract; Lanes 7, 8 and 9: 10, 5, and 2.5 μg bovine mitochondrial extract.
• Reduced vertical streaking—Coomassie G-250 dye binds to nonionic detergent molecules in the sample and carries them in the dye front, ahead of resolving proteins • Better separation of proteins—positively charged proteins with high isoelectric points are converted to proteins with a net negative charge, allowing migration to the anode
Recommended products NativeMark Unstained Protein Standard is recommended for use with native gel chemistries, including our Tris-glycine, Tris-acetate, and NativePAGE gel systems. This standard offers a wide molecular weight range of 20–1,200 kDa, and the 242 kDa β-phycoerythrin band is visible as a red band after electrophoresis for reference (prior to staining). See page 40 for details.
• Minimized protein aggregation—Coomassie G-250 dye binding allows separation of membrane proteins and proteins with exposed hydrophobic areas
Learn more at thermofisher.com/nativepage For ordering information refer to page 84. For quick reference on the protocol please refer to page 78.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Novex Tricine gels
19
Novex Tricine gel
High-resolution gels for peptide analysis and low molecular weight proteins The Invitrogen™ Novex™ Tricine gel system is a modification of the Tris-glycine system in which tricine replaces glycine in the running buffer. This system uses a discontinuous buffer system specifically designed for the resolution of low molecular weight proteins (Figure 14). Advantages of Novex Tricine gels over Tris-glycine gels include: • Increased resolution of proteins with molecular weights as low as 2 kDa (Figure 15)
Specifications • Shelf life: 1–2 months • Average run time: 90 minutes
• Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per 10-well gel: 25 μL
In the traditional Tris-glycine protein gel system, the resolution of smaller proteins (<10 kDa) is hindered by the continuous accumulation of free dodecyl sulfate (DS) ions from the SDS sample and running buffers in the stacking gel, which causes mixing of the DS ions with smaller proteins and results in fuzzy bands and decreased resolution. The mixing also interferes with the fixing and staining of smaller proteins. The Novex Tricine gel system uses a low pH in the gel buffer and sub-
Figure 15. Novex Tricine gel migration chart. For optimal resolution, protein bands should migrate within the shaded areas.
ts acquired w sul ith Re
• Minimized protein modification due to the lower pH of the tricine buffering system
How Novex Tricine gels work
Sample preparation is not the only factor that can result in poorly resolved bands. You can minimize protein degradation by using gels with neutral-pH chemistry.
• Polyacrylamide concentrations: fixed 10% and 16%; gradient 10–20%
• Improved compatibility with direct protein sequencing applications after transferring to PVDF membranes
Good to know
Did you know?
• Separation range: 2–20 kDa
18
th e
Mini Gel Tan
k
Figure 14. Novex Tricine gel electrophoresis. Protein standards and samples were loaded at 10 μL sample volumes on Invitrogen™ Novex™ 10–20% Tricine Protein Gels. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands were observed after staining with SimplyBlue SafeStain. Images were acquired using a flatbed scanner. Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 μg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 μg HeLa cell lysate; Lane 5: 20 μg HeLa cell lysate; Lane 6: 5 μg BSA; Lane 7: 40 μg Jurkat cell lysate; Lane 8: 5 μg GST fusion protein; Lane 9: Novex Sharp Unstained Protein Standard; Lane 10: 5 μg β-galactosidase.
stitutes tricine for glycine in the running buffer. The smaller proteins and peptides that migrate with the stacked DS ions
Recommended products
in the Tris-glycine gel system are well separated from DS ions
Use Novex Tricine gels with our In-Gel Tryptic Digestion Kit for separation and digestion of peptides for mass spectrometry.
in the Novex Tricine gel system, offering sharper bands and higher resolution.
Learn more at thermofisher.com/tricine For ordering information refer to page 85. For quick reference on the protocol please refer to page 79.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Novex IEF gels
21
Novex IEF gel
Precast gels for isoelectric point determination
Separated on precast vertical gel (slab) Cathode –
Isoelectric focusing (IEF) is an electrophoresis technique that separates proteins based on their isoelectric point (pI). The pI is the pH at which a protein has no net charge and does not move in an electric field. Invitrogen™ Novex™ IEF gels effectively create a pH gradient so proteins separate according to their unique pI (Figure 16 and 17). These gels can be used for pI determination or for detection of minor changes in a protein due to deamination, phosphorylation, or glycosylation, and can resolve different proteins of similar size that cannot be resolved on standard SDS-PAGE gels.
• Shelf life: 2 months
Did you know?
• Average run time: 2.5 hours
Harry Svensson-Rilbe and his student Olof Vesterberg first described the theory of separation of amphoteric proteins along a pH gradient by applying an electric field in the 1960s.
• Separation range: —pH 3–10 gels: pI performance range is 3.5–8 —pH 3–7 gels: pI performance range is 3.0–7.0 • Polyacrylamide concentration: fixed 5% • Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per 10-well gel: 20 μL
pl
1
2
3
4
5
6
7
8
9
• Higher resolution of slight differences in size when used in combination with SDS-PAGE for 2D electrophoresis
Anode +
10
8.0
• Accurate pI determination • Clear, sharp bands for easy identification of protein modifications
ts acquired w sul ith Re
When used with our convenient, pre-optimized buffers, solubilizers, and molecular weight markers, Novex IEF gels can provide:
Specifications
20
7.4
th e
Mini Gel Ta
nk
Figure 16. Novex IEF gel electrophoresis. A 2-fold dilution series 6.0 of IEF Marker 3–10 was run in duplicate on an Invitrogen™ 4.5 Novex™ pH 3–10 IEF Protein Gel using a Mini Gel Tank. The IEF Marker 3–10 consists of proteins with a variety of isoelectric points; these proteins include lectin (pI = 7.8, 8.0, and 8.3), myoglobin from horse muscle (pI = 6.9 and 7.4), carbonic anhydrase from bovine erythrocytes (pI = 6.0), β-lactoglobulin from bovine milk (pI = 5.2 and 5.3), soybean trypsin inhibitor (pI = 4.5), and glucose oxidase (pI = 4.2). After electrophoresis, the gel was fixed and stained using Coomassie R-250 dye. Gel imaging was performed with a flatbed scanner. Volume of IEF Marker 3–10 loaded: Lanes 1 and 6: 20 μL; Lanes 2 and 7: 10 μL; Lanes 3 and 8: 5 μL; Lanes 4 and 9: 2.5 μL; Lanes 5 and 10: blank.
Figure 17. Novex IEF gel migration chart using the Novex IEF marker. Proteins shown are 1: amyloglucosidase (Aspergillus niger), pI = 3.5; 2: glucose oxidase (Aspergillus niger), pI = 4.2; 3: trypsin inhibitor (soybean), pI = 4.5; 4a and 4c: β-lactoglobulin (bovine, milk), pI = 5.2 and 5.3; 5: carbonic anhydrase (bovine, erythrocytes), pI = 6.0; 6a and 6c: myoglobin (horse, muscle), pI = 6.9 and 7.4; 7a, 7m and 7c: lectin (Lens culinaris), pI = 7.8, 8.0 and 8.3; 8: ribonuclease A (bovine, pancreas), pI = 9.5; and 9: cytochrome c (horse, heart), pI = 10.7.
Recommended products Novex IEF buffer kits—includes optimized cathode, anode, and sample buffers to reduce variability and enable consistent results. IEF Marker 3–10—ready to use, enables accurate results.
ZOOM™ IEF Fractionator Combo Kit— offers a fast, reliable method to reduce sample complexity, enrich low-abundance proteins, and increase the dynamic range of detection.
Learn more at thermofisher.com/ief For ordering information refer to page 85. For quick reference on the protocol please refer to page 79.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Choose the electrophoresis chamber system and power supply
Select the standard
Novex Zymogram gels
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
23
Table 1. Novex Zymogram gels available. Novex Zymogram gel Novex Zymogram gelatin gel
Easy in-gel protease analysis Invitrogen™ Novex™ Zymogram gels are excellent tools for detecting and characterizing proteases that utilize casein or gelatin as a substrate. Casein and gelatin are the most commonly used substrates for demonstrating the activity of proteases. Novex Zymogram gels are used to analyze a variety of enzymes, including matrix metalloproteinases, lipases, and other proteases (Figure 18). Available gel types are shown in Table 1.
Novex Zymogram casein gel
Novex Zymogram blue casein gel
Gel composition
10% TrisGlycine gel
12% TrisGlycine gel
4–16% TrisGlycine gel
Substrate
0.1% gelatin
0.05% casein
0.1% casein, with blue stain incorporated in gel
Protease analysis 10% gel (w/gelatin)
12% gel (w/casein)
4—16% gel (w/prestained casein blue)
10
20
Sensitivity
10–6 units of collagenase
7 x 10 –4 units of trypsin
1.5 x 10 –3 units of trypsin
How do Novex Zymogram gels work?
Post-staining required?
Yes
Yes
No
Protease samples are denatured in SDS buffer under nonre-
Separation range
20–120 kDa
30
Good to know
1
40
30–150 kDa
10–220 kDa
Zymogram gel using Novex Tris-Glycine SDS Running Buffer. After electrophoresis, the enzyme is renatured by incubating the gel in Invitrogen™ Novex™ Zymogram™ Renaturing Buffer
Specifications
that contains a nonionic detergent. The gels are then equili-
• Shelf life: 2 months
brated in Invitrogen™ Novex™ Zymogram™ Developing Buffer to add divalent metal cations required for enzymatic activity, and then stained and destained. Regions of protease activity appear as clear bands against a dark blue background where the protease has digested the substrate.
% of length of gel
ducing conditions and without heating, and run on a Novex
50 2 1
60
2
2
3 3
• Average run time: 90 minutes • Separation range: 10–220 kDa (Figure 19) • Polyacrylamide concentrations: fixed 10% (with gelatin), fixed 12% (with casein); gradient 4–16% (with blue casein)
70
Figure 19. Novex Zymogram gel migration chart. The numbered bands refer to the following proteases: Band 1: Collagenase Type I (140 kDa) Band 2: Thermolysin (37 kDa) Band 3: Chymotrypsin (30 kDa) Band 4: Trypsin (19 kDa)
4
3
80
4
2
• Gel dimensions: 8 x 8 cm (1 mm thick) • Maximum sample volume per well: 20 μL
2
3
4
5
6
7
8
9 10
4
90
ts acquired w sul ith Re
1
22
Figure 18. Novex Zymogram gel electrophoresis. Type I collagenase was run in duplicate on an Invitrogen™ Novex™ 10% Zymogram (Gelatin) Protein th e k Gel using a Mini Gel Tank. The gel was developed Mini Gel Tan using Novex Zymogram Renaturing Buffer and Novex Zymogram Developing Buffer and stained using SimplyBlue SafeStain. Images were acquired using a flatbed scanner. Lanes 3 and 7: 5 μL of 2.0 μU/mL type I collagenase; Lanes 1, 4, 5, and 10: 12 μL SeeBlue Prestained Protein Standard.
4
100
Recommended products After electrophoresis, incubate the gel in Zymogram Renaturing Buffer to renature the enzyme. The gels are then equilibrated in Zymogram Developing Buffer to add divalent metal cations required for enzymatic activity.
Learn more at thermofisher.com/zymogram For ordering information refer to page 85. For quick reference on the protocol please refer to page 80.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
24
Prepare samples and select buffers
Select precast gel
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
E-PAGE High-Throughput Precast Gel System
E-PAGE gel
E-PAGE 48 8% Gel
Protein separation and analysis for increased sample throughput
E-PAGE 96 6% Gel
0%
0% 220 kDa
10%
The Invitrogen E-PAGE High-Throughput Precast Gel System is designed for fast, bufferless medium- and high-throughput protein analysis. Invitrogen™ E-PAGE™ 48-well and 96-well precast gels consist of a buffered gel matrix and electrodes packaged inside a disposable, UV-transparent cassette. Each cassette is labeled with a unique barcode to facilitate identification of the gel using commercial barcode readers. These gels can be loaded by multichannel pipettor or automated loading system. The E-PAGE system also includes E-Base™ integrated devices to run the gels, an E-Holder™ platform for optional robotic loading, and free E-Editor™ 2.0 Software to align images for easy comparison. ™
220 kDa
™
Advantages of using the E-PAGE High-Throughput Precast Gel System include: • Ease-of-use—quick setup and fast protein separation in about 23 minutes
25
120 kDa
25%
Did you know?
20% 120 kDa 30%
100 kDa
60 kDa 40 kDa
80 kDa
75% 20 kDa
40%
Specifications
50%
60 kDa
• Shelf life: 6 months
50%
• Average run time: 14 minutes
50 kDa
100%
Figure 21. E-PAGE gel migration chart. Migration patterns of the Invitrogen™ E-PAGE™ MagicMark™ Unstained Protein Standard are shown.
Our E-Base devices are compatible with the Society for Biomolecules Screening (SBS) standard plate size and can be conveniently mounted on liquid handling robot decks.
40 kDa 60%
• Separation range: 10–200 kDa
30 kDa
• Polyacrylamide concentrations: –– E-PAGE™ 48 gel: fixed 8%
70% 20 kDa
–– E-PAGE™ 96 gel: fixed 6%
80%
• Gel dimensions: 13.5 x 10.8 cm (3.7 mm thick) • Maximum sample volume per well: –– E-PAGE 48 gel: 20 µL –– E-PAGE 96 gel: 15 µL
90%
100%
B
A
• Fast loading—compatible with multichannel pipettors and robotic loading
Recommended products
• Efficient western blotting and staining—optimized protocols and reagents
The E-PAGE™ SeeBlue™ Prestained Protein Standard or E-PAGE MagicMark Unstained Protein Standard are specifically designed for use with E-PAGE gels.
Good to know
C Mother E-Base
E-PAGE 96 gels
How do E-PAGE gels work? E-PAGE gels run in the Invitrogen™ E-Base electrophoresis de-
Daughter E-Base
vice, which has an integrated power supply for direct connection to an electrical outlet. Use the Invitrogen™ Mother E-Base™ device for a single E-PAGE gel, or use the Mother E-Base device in conjunction with two or more Invitrogen™ Daughter
Figure 20. Loading and running E-PAGE gels. (A) Loading E-PAGE 48 gels using a multi-channel pipettor. (B) Loading E-PAGE 96 gels using a multi-channel pipettor. (C) The Mother/Daughter E-Base combination.
E-Base™ devices for running multiple gels simultaneously.
Learn more at thermofisher.com/epage For ordering information refer to page 85.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Prepare the sample Sample prep kits
High salt concentration in samples: High salt concentrations result in increased conductivity that affects protein migration, and
Before a sample can be loaded onto a gel for analysis, it must be properly prepared. Depending on the gel type, this may involve denaturing the proteins, reducing any disulfide bonds, adjusting the ionic strength, and removing interfering contaminants. General guidelines for preparing samples are provided below. General guidelines for preparing samples: Prepare your sample in the appropriate sample buffer such that the final concentration of the sample buffer is 1X. Recommended sample buffers are listed on page 29. Running reduced and non-reduced samples: For optimal
Protein gel electrophoresis technical handbook
Post stain
can result in gel artifacts in adjacent lanes containing samples with normal salt concentrations. Perform dialysis or precipitate and resuspend samples in lower-salt buffer prior to electrophoresis.
Pierce SDS-PAGE Sample Prep Kit
Sample treated with the Pierce SDS-PAGE Sample Prep Kit
Untreated M
S
M
S
M
S
M
Guanidine-HCl in samples: Samples solubilized in guanidineHCl have high ionic strength, and produce increased conductivity similar to high salt concentrations. In addition, guanidine precipitates in the presence of SDS leading to various types of gel artifacts. If possible, change the solubilization agent by dialysis prior to electrophoresis.
Cell lysates Consider the following when performing electrophoresis of cell lysates: • Genomic DNA in the cell lysate may cause the sample to become viscous and affect protein migration patterns and resolution. Shear genomic DNA to reduce viscosity before loading the sample. • Cells lysates contain soluble and insoluble fractions. The size of each fraction depends on the type of sample being analyzed. The nature of the insoluble fraction may result in altered protein migration patterns and resolution. Separate the two fractions by centrifugation and load them on separate lanes for electrophoresis. • If radioimmunoprecipitation assay (RIPA) buffer is used in cell lysis, subsequent blotting of proteins less than 40 kDa may be inhibited due to the presence of Triton™ X-100 in the buffer.
A protein sample can be purged of any contaminants typically in only 10 minutes using the Thermo Scientific™ Pierce™ SDS-PAGE Sample Prep Kit. This is much faster than dialysis or ultrafiltration and yields higher protein recoveries while concentrating the sample. Advantages of using the Pierce SDS-PAGE Sample Prep Kit include: • Eliminates artifacts caused by incompatible contaminants—removes dyes, reducing agents, detergents, sugars, glycerol, guanidine, urea, and ammonium sulfate to provide reproducible results for SDS-PAGE analysis (Figure 22) • Compatible with the Thermo Scientific™ Pierce™ BCA Assay—allows quantification of the processed sample • Enriches dilute protein solutions—concentrates protein sample by eight-fold in less than 20 minutes for SDS-PAGE analysis (Figure 22) • Fast and easy to use for up to 70 μg of protein per sample—uses new spin cup format that allows higher amounts of protein to be processed than with the original procedure
Figure 22. Minimize distortion caused by detergents. Rat C6 cells were lysed and a membrane protein fraction isolated using the Thermo Scientific™ MemPER™ Eukaryotic Membrane Protein Extraction Reagent. Membrane and hydrophilic cell fractions were separated by SDS-PAGE using 4–20% gradient gels with or without prior treatment using the Pierce SDS-PAGE Sample Prep Kit. Western blot analysis was performed using an antibody against cytochrome oxidase subunit 4 (COX4) and Thermo Scientific™ SuperSignal™ West Femto chemiluminescent substrate. Kit-treated samples exhibit better band straightness and resolution with low molecular weight proteins than samples that were untreated. S = Soluble fraction (hydrophilic) M = Membrane fraction 100
Figure 23. Consistent 88% 80 protein 85% 77% 77% recovery is 75% 74% achieved 60 using the Pierce SDS40 PAGE Sample Prep Kit. 20 Pure proteins (60 µg) of 0 assorted Carbonic Ovalbumin Transferrin Ubiquitin Cytochrome c Bacterial anhydrase lysat e molecular mass (30, 44, 80, 86, and 120 kDa) as well as a bacterial lysate were processed using this kit. Protein concentrations were determined with the Pierce BCA Protein Assay Kit and reported as percent protein recovered. Table 2. Interfering substances effectively removed.
Good does to know How it work?
For quick protein clean-up and enrichment for SDS-PAGE we
samples on the same gel. If you do choose to run reduced and
recommend using the Thermo Scientific Pierce SDS-PAGE Sample
non-reduced samples on the same gel, do not run reduced and
Prep Kit, which removes substances such as guanidine-HCL
Our Pierce SDS-PAGE Sample Prep Kit uses a unique resin
non-reduced samples in adjacent lanes. The reducing agent may
and ionic detergents that can result in protein bands that appear
of modified diatomaceous earth that binds protein in DMSO.
have a carry-over effect on the non-reduced samples if they are in
smeared or wavy in the gel or on a western blot.
Simply combine 2–300 μL of sample containing up to 70 μg of
Interfering reagents
Percent protein recovered (Starting amount = 20 µg BSA)
Control (water)
75%
0.5 M Sodium chloride
80%
2 M Ammonium sulfate
76%
protein with 20 μL of Pierce™ SDS Protein Binding Resin and
20% SDS
75%
DMSO. After the proteins bind to the resin, wash away the
10% Triton™ detergent
75%
unbound contaminating chemicals. Finally, elute the sample
6 M Urea:DMSO (1:3 ratio)
75%
buffer results in proteolysis (Kubo, 1995). We recommend heating
in 50 μL of the Elution Buffer. The recovered protein sample is
1 M Sodium chloride
75%
samples for denaturing electrophoresis (reduced or non-reduced)
ready to mix with the supplied Sample Loading Buffer for
6 M Urea
74%
at 85°C for 2–5 minutes for optimal results. Do not heat the
gel loading.
10% CHAPS
80%
25% Glycerol
71%
10% OTG
71%
2 M Guanidinium•HCl
70%
40% Sucrose
70%
Heating samples: Heating the sample at 100°C in SDS-containing
samples for non-denaturing (native) electrophoresis or Novex Zymogram Gels.
Learn more at thermofisher.com/PAGEsampleprep
For ordering information refer to page 85.
S
Quick protein clean-up and enrichment for SDS-PAGE
results, we do not recommend running reduced and non-reduced
close proximity.
27
Percent protein recovered
26
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
28
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Select buffers
29
Recommended SDS-PAGE buffers and reagents
Buffers and reagents
Protein samples prepared for PAGE analysis are denatured by heating in the presence of a sample buffer with or without a reducing agent. The protein sample is mixed with the sample buffer and heated for 2–10 minutes, then cooled to room temperature before it is applied to the sample well on the gel. Loading buffers also contain glycerol so that they are heavier than water and sink neatly to the bottom of the buffer-submerged well when added to a gel.
Protein gel electrophoresis technical handbook
Post stain
Gel type
If suitable, negatively charged, low molecular weight dye is also included in the sample buffer; it will migrate at the buffer front, enabling one to monitor the progress of electrophoresis. The most common tracking dye for sample loading buffers is bromophenol blue. We offer premixed, reliable SDS-PAGE buffers and reagents including sample buffers, running buffers, reducing agents, and antioxidants.
Learn more at
Bolt Bis-Tris Plus Gel
Sample buffer optimized for use with the gel
Other compatible sample buffers
Running buffer optimized for use with the gel
• Bolt™ Sample Reducing Agent (10X) • Bolt™ LDS Sample Buffer (4X) (nonreducing) • Bolt Antioxidant
• Pierce™ LDS Sample Buffer (4X) for storage at RT
• Bolt™ MES SDS Running Buffer (20X) • Bolt™ MOPS SDS Running Buffer (20X)
NuPAGE Bis-Tris Gel
• NuPAGE Sample Reducing Agent (10X) • NuPAGE Antioxidant • NuPAGE™ LDS Sample Buffer (4X) (nonreducing)
NuPAGE Tris-Acetate Gel
• Novex Tris-Glycine SDS Sample Buffer (2X) • NuPAGE Sample Reducing Agent (10X) • Novex Tris-Glycine Native Sample Buffer (2X)
Novex Tris-Glycine Gel
• Novex Tris-Glycine SDS Sample Buffer (2X) • NuPAGE Sample Reducing Agent • Novex Tris-Glycine Native Sample Buffer (2X)
™
• Pierce™ Lane Marker Non• NuPAGE™ MES SDS Reducing Sample Running Buffer (20X) Buffer (5X)— • NuPAGE™ MOPS SDS storage at RT; Running Buffer (20X) when you desire to dilute your sample less and require transferable marker dye to nitrocellulose membranes • Pierce™ Lane Marker Reducing Sample Buffer (5X)—when you desire to dilute your sample less and require transferable marker dye to nitrocellulose membranes
MES vs. MOPS Running Buffer: • Use MES SDS running buffers to resolve small molecular weight proteins. • Use MOPS running buffers to resolve mid-size proteins. MES has a lower pKa than MOPS, enabling gels with MES running buffer to run faster than gels with MOPS SDS running buffer. The difference in ion migration affects stacking and results in a difference in protein separation range between these buffers.
• NuPAGE™ Tris-Acetate SDS Running Buffer (20X) • Novex Tris-Glycine Native Running Buffer (10X)
Reducing agent: When preparing samples for reducing gel electrophoresis, any of the following reducing agents may be used: •B olt Sample Reducing Agent
• Novex Tris-Glycine SDS Running Buffer (10X) • Novex Tris-Glycine Native Running Buffer (10X) • Pierce™ Tris-Glycine SDS Buffer (10X) • BupH™ Tris-Glycine-SDS Buffer Packs
•N uPAGE Sample Reducing Agent
Novex Tricine Gel
• Novex™ Tricine SDS Sample Buffer (2X)
• Novex Tricine SDS Running Buffer (10X)
NativePAGE Gel
• NativePAGE™ Sample Buffer (4X) • NativePAGE™ 5% G-250 Sample Additive
• NativePAGE™ Running Buffer (20X) • NativePAGE™ Cathode Buffer Additive (20X)
Novex IEF Gel
• Novex™ IEF Sample Buffer, pH 3–10 (2X) • Novex™ IEF Sample Buffer, pH 3–7 (2X)
• Novex™ IEF Anode Buffer (50X) • Novex™ IEF Cathode Buffer, pH 3–10 (10X) • Novex™ IEF Cathode Buffer, pH 3–7 (10X)
Novex Zymogram Gels*
• Novex Tris-Glycine SDS Sample Buffer (2X)
• Novex Tris-Glycine SDS Running Buffer (10X)
ithiothreitol (DTT), 50 mM final D concentration • β-mercaptoethanol (β-ME), 2.5% final concentration • tris(2-carboxyethyl)phosphine (TCEP), 50 mM final concentration Add the reducing agent to the sample up to an hour before loading the gel. Avoid storing reduced samples for long periods, even if they are frozen. Reoxidation of samples can occur during storage and produce inconsistent results.
*Novex Zymogram Developing Buffer (10X) and Novex Zymogram Renaturing Buffer (10X) are available for visualizing the Novex Zymogram gels.
thermofisher.com/electrophoresisbuffers For ordering information refer to page 85.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
30
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
31
Buffer recipes NuPAGE buffer recipes
Tris-glycine buffer recipes
Buffer
Storage
NuPAGE LDS Sample Buffer
+4˚–25˚C
NuPAGE MOPS SDS Running Buffer*
Component
Concentration (1X)
Glycerol Tris base Tris HCl LDS EDTA SERVA™ Blue G-250 Phenol Red
0% 141 mM 106 mM 2% 0.51 mM 0.22 mM 0.175 mM (pH 8.5)
NuPAGE Tris-Acetate SDS Running Buffer
Tris-Glycine SDS Sample Buffer
+4˚C
Tris-Glycine Native Sample Buffer
50 mM 50 mM 0.1% 1 mM (pH 7.7)
Tris-Glycine SDS Running Buffer
50 mM 50 mM 0.1% 1 mM (pH 7.3)
Tris-Glycine Native Running Buffer
Room temperature
Room temperature
+4˚–25˚C Bicine Bis-Tris (free base) EDTA Chlorobutanol
25 mM 25 mM 1.0 mM 0.05 mM (pH 7.2)
Tris base Tricine SDS
50 mM 50 mM 0.1% (pH 8.24)
Tris-Glycine Transfer Buffer
Component
Concentration (1X)
Tris HCl* Glycerol SDS Bromophenol Blue Deionized water
63 mM 10% 2% 0.0025% — (pH 6.8)
Tris HCl* Glycerol Bromophenol Blue Deionized water
100 mM 10% 0.0025% — (pH 8.6)
Tris base Glycine SDS Deionized water
25 mM 192 mM 0.1% — (pH 8.3)
Tris base Glycine Deionized water
25 mM 192 mM — (pH 8.3)
Tris base Glycine Deionized water
12 mM 96 mM — (pH 8.3)
+4˚C
+4˚–25˚C MES Tris base SDS EDTA
NuPAGE™ Transfer Buffer
Storage
+4˚–25˚C MOPS Tris base SDS EDTA
NuPAGE MES SDS Running Buffer*
Buffer
Room temperature
+4˚–25˚C * Tris HCl solutions are prepared from Tris base and pH adjusted with 6 N HCl.
* The pre-mixed buffers (Cat. Nos. NP0001 and NP0002) also contain trace amounts of the proprietary NuPAGE Antioxidant (Cat. No. NP0005) for stability. Additional Antioxidant may be required with specific protocols.
For ordering information refer to page 85.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
32
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
33
Buffer recipes Isoelectric focusing buffer recipes
Tricine buffer recipes Buffer
Storage
Tricine SDS Sample Buffer
+4˚C
Tricine SDS Running Buffer
Room temperature
Component
Concentration (1X)
Tris HCl* Glycerol SDS Coomassie Blue G Phenol Red Deionized water
450 mM 12% 4% 0.0075% 0.0025% – (pH 8.45)
Tris base Tricine SDS Deionized water
100 mM 100 mM 0.1% – (pH 8.3)
Buffer
Storage
IEF Sample Buffer pH 3-7
+4˚C
IEF Sample Buffer pH 3-10
+4˚C
* Tris HCl solutions are prepared from Tris base and pH adjusted with 6 N HCl.
IEF Cathode Buffer pH 3-10 (upper buffer chamber)
+4˚C
Zymogram buffer recipes
IEF Anode Buffer (for both pH ranges) (lower buffer chamber)
Room temperature
Urea-Thiourea-CHAPS (rehydration buffer for IPG strips)
–20˚C
Storage
Zymogram Renaturing Buffer
Room temperature
Zymogram Developing Buffer
Room temperature
* Tris HCl solutions are prepared from Tris base and pH adjusted with 6 N HCl.
For ordering information refer to page 85.
Component
Concentration (1X)
Triton™ X-100 Deionized water
2.7% (w/v) in H2O
Tris HCI* NaCl CaCl2•2 H2O Brij™ 35 Deionized water
50 mM 200 mM 5 mM 0.006% (w/v) _ (pH 7.6)
Concentration (1X)
Lysine (free base)
40 mM
Glycerol Deionized water
15% —
Arginine (free base) Lysine (free base) Glycerol Deionized water
20 mM 20 mM 15% —
Lysine (free base) Deionized water
40 mM —
Arginine (free base) Lysine (free base) Deionized water
20 mM 20 mM — (pH 10.1)
Phosphoric acid 85% Deionized water
7 mM — (pH 2.4)
Deionized urea Deionized thiourea CHAPS Ampholytes* Bromophenol Blue
7M 2M 2–4% 0.2–2.0% 0.002%
Ultrapure water
—
DTT
20 mM
+4˚C
IEF Cathode Buffer pH 3-7 (upper buffer chamber)
Buffer
Component
* For ZOOM™ Strip pH 9-12 use 1% ZOOM™ Focusing Buffer pH 7-12 instead of ampholytes.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
34
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
35
Select the standard Protein ladders and standards To assess the relative molecular weights (sizes) of proteins in a sample, a mixture containing several proteins of known molecular mass are run alongside the test sample lane(s). Often these protein mixtures are run on the outer lanes of the gel, to maximize the number of remaining gel wells for test samples, but can also be useful in the middle wells of the gel when running a large gel with many wells. Such sets of known protein mixtures are called protein molecular weight markers or protein ladders. It is important to choose a protein ladder that consists of proteins with molecular weights that span the molecular weight range of the protein(s) of interest. A standard curve can be constructed from the distances
each marker protein migrates through the gel. After measuring the migration distance that an unknown protein travels through the same gel, its molecular weight can be determined graphically from the standard curve. Several kinds of ready-to-use protein molecular weight (MW) markers are available that are labeled, prestained, or unstained for different modes of detection and downstream applications. We offer ladders suitable for both SDSPAGE as well as native PAGE.
Unstained protein ladders
Ready-to-use prestained and unstained protein ladders with exceptional lot-to-lot consistency
Low range
PageRuler Unstained Low Range Protein Ladder
Broad range
PageRuler Unstained Protein Ladder
We offer a broad range of prestained and unstained protein
High range
NativeMark Unstained Protein Standard
ladders supplied in a ready-to-use format to facilitate easy protein
Recommended for: • Precise determination of target protein molecular weight
analysis during gel electrophoresis and western blotting (Table 3). All of our protein ladders offer: • Performance—sharp protein bands and consistent migration patterns provide easy molecular weight determination
Prestained protein ladders Low range
PageRuler Prestained Protein Ladder
• Convenience—protein ladders are ready to load, with no heating required
Broad range
PageRuler Plus Prestained Protein Ladder Spectra™ Multicolor Broad Range Protein Ladder
• Reliability—exceptional lot-to-lot consistency and reproducibility
High range
HiMark Prestained Protein Standard Spectra Multicolor High Range Protein Ladder
Recommended for: • Approximate determination of molecular weight • Monitoring the progress of electrophoresis runs • Estimating the efficiency of protein transfer to the membrane during western blotting
Learn more at thermofisher.com/proteinstandards
Other Western
MagicMark XP Western Protein Standard
Specialty
PageRuler Prestained NIR Protein Ladder BenchMark Fluorescent Protein Standard BenchMark His-tagged Protein Standard IEF Marker 3-10
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
36
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
37
Table 3. Protein standard selection guide Category
Range
No. of bands
Reference bands
Protein MW determination
Protein band visualization
Monitoring electrophoresis run
Coomassie dye, silver, Monitoring protein or fluorescent staining transfer
Chemiluminescent band visualization
PageRuler Unstained Low Range Protein Ladder
3.4–100 kDa
8
25 kDa
Best
NA
NA
Best
NA
Good
PageRuler Unstained Protein Ladder
10–200 kDa
14
50 kDa
Good
NA
NA
Good
NA
Good
NativeMark Unstained Protein Standard
20–1,200 kDa
8
Best for native electrophoresis
NA
NA
Best
NA
Good
PageRuler Prestained Protein Ladder
10–180 kDa
10
Green 10 kDa; orange 70 kDa
Good
Good
Good
NA
Good
Good
PageRuler Plus Prestained Protein Ladder
10–250 kDa
9
Green 10 kDa; orange 25 and 70 kDa
Good
Good
Good
NA
Good
NA
HiMark Prestained Protein Standard
30–460 kDa
9
Best for high MW proteins
Good
Good
NA
Best for high MW proteins
NA
Product
Unstained ladders and standards Unstained standards
Pretained protein ladders Prestained protein standards
Spectra Multicolor Broad Range 10–260 kDa Protein Ladder
10
Green 10 and 50 kDa; orange 40, 70, and 260 kDa; pink 140 kDa
Good
Best
Best
NA
Best
NA
Spectra Multicolor High Range Protein Ladder
40–300 kDa
8
Green 50 kDa; orange 70 and 300 kDa
Good
Best
Best
NA
Best
NA
Other ladders and standards IEF
IEF Marker 3-10
pI 3.5–10.7
13
Best for pI estimation
NA
NA
Good
NA
NA
Chemiluminescent standard
MagicMark XP Western Protein Standard
20–220 kDa
9
Good
NA
NA
Good
NA
Best
Near infrared (NIR) standard
PageRuler Prestained NIR Protein Ladder
11–250 kDa
10
Good
NA
NA
NA
NA
NA
Fluorescent standard
BenchMark Fluorescent Protein Standard
11–155 kDa
7
Good
NA
NA
NA
NA
Good
His-tag standard
BenchMark His-tagged Protein Standard
10–160 kDa
10
Best
NA
NA
Good
NA
Good for detection with anti-His antibody
55 kDa
Learn more at thermofisher.com/proteinstandards
For ordering information refer to page 86.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
38
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
39
Unstained ladders and standards
PageRuler Unstained Low Range Protein Ladder
PageRuler Unstained Protein Ladder
Sharp bands and precise molecular weight estimation for low molecular weight proteins
Sharp bands and precise molecular weight estimation for a wide range of proteins
Thermo Scientific™ PageRuler™ Unstained Low Range Protein Ladder is a mixture of eight proteins and peptides for use as size standards that resolve into clearly identifiable sharp bands when analyzed by SDS-PAGE. The proteins (except for the 5 and 3.4 kDa peptides) contain an integral Strep-tag™ II Sequence and may be detected on western blots using Strep-Tactin™ Conjugates. • Comprehensive—eight proteins and peptides spanning 3.4 to 100 kDa; the 25 kDa band is more intense than the other bands for easy orientation • Versatile—compatible with western blots by staining with Ponceau S dye or Coomassie dye; compatible with Thermo Scientific™ Pierce™ Reversible Protein Stain Kit for Nitrocellulose Membranes or other protein stains
PageRuler Unstained Low Range Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The PageRuler Unstained Protein Ladder is recommended for Novex Tris-Glycine, Bis-Tris or Tris-Acetate gels.
Thermo Scientific™ PageRuler™ Unstained Protein Ladder is a mixture of 14 recombinant, highly purified, unstained proteins for use as size standards in SDS-PAGE and western blotting. Each protein in the ladder contains an integral Strep-tag II Sequence, which can be detected directly on western blots using a Strep-Tactin Conjugate or an antibody against the Strep-tag II Sequence. • Comprehensive—14 highly purified proteins with excellent accuracy spanning 10 to 200 kDa; the ladder contains one 50 kDa reference band of higher intensity • Versatile—compatible with Coomassie dye; compatible with Pierce Reversible Protein Stain Kit for Nitrocellulose Membranes, silver staining, or western blotting
Learn more at
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The PageRuler Unstained Protein Ladder is recommended for Novex Tris-Glycine, Bis-Tris or Tris-Acetate gels.
Learn more at
thermofisher.com/unstainedstandards
For ordering information refer to page 86.
PageRuler Unstained Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
thermofisher.com/unstainedstandards
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
40
Prepare samples and select buffers
Select precast gel
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
41
Prestained ladders
NativeMark Unstained Protein Standard Convenient molecular weight estimation for native electrophoresis
kDa kDa
1,0481,048 1,2361,236 1,0481,048
720 720 480 480
720 720 242 242 480 480 146 146 242 242
The Invitrogen NativeMark Unstained Protein Standard is designed for molecular weight estimation of proteins using native gel electrophoresis. ™
™
• Comprehensive—contains a wide range of high molecular weight proteins, providing 8 protein bands in the range of 20–1,200 kDa • Versatile—can be visualized using Coomassie, silver, or fluorescent stains after electrophoresis, or with Ponceau S, Coomassie, or other membrane stains after western transfer
PageRuler Prestained Protein Ladder
kDa kDa 1,2361,236
66 66
146 146
NativeMark Unstained Protein Standard NativePAGE Bis-Tris gels
66 66 20 20 20 20
3–12% 3–12%
4–16% 4–16%
Storage specifications • Storage buffer: Bis/Tris-HCl (pH 7.0), NaCl, glycerol, and Ponceau S • Storage conditions: upon receipt store at –20°C • Stability: 6 months
Recommended products The NativeMark Unstained Protein Standard is recommended for use with NativePAGE Bis-Tris gels, Novex Tris-Glycine gels, or NuPAGE Tris-Acetate gels.
Outstanding clarity for easy molecular weight determination of low molecular weight proteins Thermo Scientific™ PageRuler™ Prestained Protein Ladder is a mixture of 10 blue-, orange-, and green-stained proteins for use as size standards in SDS-PAGE and western blotting. The mobility of prestained proteins can vary in different SDSPAGE buffer systems; however, they are suitable for approximate molecular weight determination when calibrated against unstained standards in the same system. • Comprehensive—contains 10 proteins with a range of 10 to 180 kDa; includes one 70 kDa reference protein colored with an orange dye and one 10 kDa reference protein colored with a green dye
PageRuler Prestained Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The PageRuler Prestained Protein Ladder is recommended for use with Tris-glycine, Bis-Tris, and Tris-acetate gels.
• Versatile—compatible with Coomassie dye staining and western blotting
Learn more at
Learn more at
thermofisher.com/prestainedstandards
thermofisher.com/unstainedstandards
For ordering information refer to page 86.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
42
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
PageRuler Plus Prestained Protein Ladder Outstanding clarity for easy molecular weight determination of a broad range of proteins
Protein gel electrophoresis technical handbook
Post stain
HiMark Prestained Protein Standard PageRuler Plus Prestained Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
• Comprehensive—9 proteins with a broad range of 10 to 250 kDa; includes 70 kDa and 25 kDa reference proteins that are colored with an orange dye and one 10 kDa reference protein that is colored with a green dye
kDa
198
220
98
268 238
62
171
117
• Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C
110 80
60
50
60 40 30
40
28
20
71
17
15 HiMark Prestained Protein Standard NuPAGE 3–8%10Tris-acetate SDS buffer 30
14
Standard
160
80
50
38
™
260
120 100
49
The Invitrogen HiMark Prestained Protein Standard is designed for analysis of high molecular6 3 weight proteins on NuPAGE Tris-acetate gels.
Storage specifications
kDa
kDa
460
Superb analysis of high molecular weight proteins ™
Thermo Scientific™ PageRuler™ Plus Prestained Protein Ladder is a mixture of 9 blue-, orange-, and green-stained proteins for use as size standards in SDS-PAGE and western blotting. The mobility of prestained proteins can vary in different SDS-PAGE buffer systems; however, they are suitable for approximate molecular weight determination when calibrated against unstained standards in the same system.
kDa
43
55
20
41
3.5
31
SeeBlue® Plus2
HiMark™ Pre-stained
MagicMark™ XP
Novex® Sharp Pre-stained
• Comprehensive—contains a wide range of high molecular Cat. No. LC5800 LC5602 LC5925 LC5699 weight proteins, providing 9 protein bands in the range of NuPAGE 3–8% NuPAGE NuPAGE NuPAGE Bis-Tris Gel, Storage Tris-Acetatespecifications Gel blotted to 4–12% Bis-Tris 4–12% Bis-Tris w/ Tris-acetate nitrocellulose, Gel w/ MES Gel w/ MES 30–460 kDa SDS buffer detected w/ SDS buffer SDS buffer WesternBreeze • Storage buffer: Tris-HCl, formamide, SDS, and phenol red Chemiluminescent Kit • Versatile—easy visualization of band migration during • Storage conditions: upon receipt store at –20°C electrophoresis and rapid evaluation of western transfer efficiency • Stability: 6 months from date of receipt ®
®
®
®
®
• Stability: 1 year from date of receipt
Recommended products Recommended products
The HiMark Prestained Protein Standard is recommended for use with NuPAGE Tris-Acetate gels under denaturing conditions. This standard can also be used with NuPAGE 4–12% Bis-Tris gels with Invitrogen™ NuPAGE MOPS SDS Running Buffer and Novex 4% Tris-Glycine gels. However, to obtain the best results with high molecular weight proteins, always use NuPAGE Tris-Acetate gels.
The PageRuler Plus Prestained Protein Ladder is recommended for Trisglycine, Bis-Tris, and Tris-acetate gels.
• Versatile—compatible with Coomassie dye staining and western blotting
Learn more at
The HiMark Prestained Protein Standard is also available as part of the following kits that include gels, running and sample buffers, and stains or blotting materials: • Invitrogen™ NuPAGE™ Large Protein Staining Kit • Invitrogen™ NuPAGE™ Large Protein Sensitive Staining Kit • Invitrogen™ NuPAGE™ Large Protein Blotting Kit
thermofisher.com/prestainedstandards
Learn more at thermofisher.com/prestainedstandards
For ordering information refer to page 86.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
44
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Spectra Multicolor Broad Range Protein Ladder
Spectra Multicolor High Range Protein Ladder
Superior visualization and analysis of a broad range of proteins
Superior and convenient visualization of high molecular weight proteins
Thermo Scientific Spectra Multicolor Broad Range Protein Ladder is a 4-color protein standard containing 10 prestained proteins for use in gel electrophoresis and western blotting. This standard is designed for monitoring the progress of gels during SDS-PAGE and for assessing western blot transfer efficiency. Four different chromophores (blue, orange, green, and pink) are bound to the different component proteins, producing a brightly colored ladder with an easy-to- pattern. ™
Spectra Multicolor Broad Range Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
™
• Comprehensive—10 proteins with similar intensity spanning a broad range of 10 to 260 kDa • Versatile—compatible with Coomassie dye staining and western blotting
Thermo Scientific Spectra Multicolor High Range Protein Ladder is a mixture of 8 blue-, green-, and orange-stained proteins for use as size standards for high molecular weight proteins in gel electrophoresis and western blotting. This marker is designed for monitoring the progress of gels during SDS-PAGE, assessing western blot transfer efficiency, and estimating the approximate size of proteins after gel staining or western blotting. ™
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The Spectra Multicolor Broad Range Protein Ladder is recommended for Tris-glycine, Bis-Tris, and Tris-acetate gels.
™
• Comprehensive—8 proteins of similar intensity spanning a range of 40 to 300 kDa; 3 different chromophores (blue, orange, and green) are bound to the different component proteins, producing a brightly colored ladder with an easy-to- pattern
Spectra Multicolor High Range Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The Spectra Multicolor High Range Protein Ladder is recommended for Tris-glycine, Bis-Tris, and Tris-acetate gels.
• Versatile—compatible with Coomassie dye staining and western blotting
Learn more at
Learn more at
thermofisher.com/prestainedstandards
For ordering information refer to page 86.
45
thermofisher.com/prestainedstandards
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
46
Select precast gel
Prepare samples and select buffers
Choose the electrophoresis chamber system and power supply
Select the standard
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
47
Other ladders and standards
MagicMark XP Western Protein Standard kDa
kDa
PageRuler Prestained NIR Protein Ladder
kDa
kDa
460
198
220
98
268 238
62
171
Accurate molecular weight estimation directly on western blots
260 160
120 100
49
117
80
110 80
60
50
60 40
50
38
30
40
28
MagicMark XP Western Protein Standard 20 NuPAGE Bis-Tris gel, blotted to nitrocellulose, 15 and detected with Invitrogen™ WesternBreeze™ 10 Chemiluminescent Kit
71
17
30
14
55
The MagicMark™ XP Western Protein Standard 20 6 3.5 is specifically designed for easy and convenient 41 3 protein molecular weight estimation directly on 31 HiMark SeeBlue MagicMark XP Novex Sharp Standard Pre-stained western blots. Each recombinant protein Plus2 in the Pre-stained Cat. No. LC5800 LC5602 LC5925 LC5699 Storage specifications standard contains an IgG binding site, which NuPAGE 3–8% NuPAGE NuPAGE NuPAGE Bis-Tris Gel, Tris-Acetate Gel blotted to 4–12% Bis-Tris 4–12% Bis-Tris binds the primary or secondary antibody used w/ Tris-acetate • nitrocellulose, Gel w/ MES Gel w/ MES S torage buffer: Tris-HCl (pH 6.8), DTT, glycerol, SDS, and detected w/ SDS buffer SDS buffer WesternBreeze for detection of the target protein, allowing direct SDS buffer Chemiluminescent bromophenol blue Kit visualization of the standard on the western blot. ™
®
®
®
®
™
®
®
®
• Storage conditions: upon receipt store at –20°C
• Comprehensive—consists of 9 recombinant proteins from 20 to 220 kDa • Versatile—compatible with chemiluminescent, chromogenic, and fluorescent detection
• Stability: 4 months from date of receipt
Recommended products The MagicMark XP Western Protein Standard is compatible with a broad range of gels—NuPAGE Bis-Tris gels, Novex Tris-Glycine gels, Novex Tricine gels, NuPAGE Tris-Acetate gels, and Bolt Bis-Tris Plus gels.
Sharp prestained standard for near-IR fluorescent visualization and protein sizing Thermo Scientific™ PageRuler™ Prestained NIR Protein Ladder is a mixture of 10 proteins that are stained blue and labeled with a fluorophore for near-infrared (NIR) fluorescent visualization and protein sizing following electrophoresis. The molecular weight markers in this ladder resolve into sharp bands when analyzed by SDS-PAGE. The 55 kDa band is of greater intensity and serves as a reference band. • Comprehensive—10 protein bands spanning 11 to 250 kDa • Versatile—visualize using instruments equipped for detection of near-infrared fluorescence such as certain Typhoon™ Imagers and the LI-COR Odyssey™ Infrared Imaging System; bands are directly visible because the proteins are prestained blue
Learn more at
Visual detection
Infrared detection
Storage specifications • Storage buffer: Tris-H3PO4, EDTA, SDS, DTT, sodium azide, bromophenol blue, and glycerol • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Recommended products The PageRuler Prestained NIR Protein Ladder is recommended for visual detection, infrared imaging detection, and western blotting.
Learn more at
thermofisher.com/westernblotstandard
For ordering information refer to page 86.
PageRuler Prestained NIR Protein Ladder NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
thermofisher.com/specialtystandards
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
48
Prepare samples and select buffers
Select precast gel
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
BenchMark Fluorescent Protein Standard
BenchMark His-tagged Protein Standard
Efficient estimation of molecular weight by fluorescent detection
Convenient detection and protein sizing of His-tagged proteins
BenchMark Fluorescent Protein Standard NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
The Invitrogen BenchMark Fluorescent Protein Standard consists of Alexa Fluor™ 488 dye– conjugated proteins for molecular weight estimation of fluorescently labeled proteins. ™
Storage specifications
The Invitrogen BenchMark His-tagged Protein Standard can be used as a positive control and for molecular weight sizing in His-tagged fusion protein detection. Each protein in the standard has a 6xHis tag.
• Storage buffer: Tris-HCl, SDS, glycerol, and Coomassie Blue G-250
• Comprehensive—10 sharp and clear bands from 10 to 160 kDa for molecular weight estimation of His-tagged proteins
™
• Comprehensive—consists of 7 distinct protein bands in the range of ~11–155 kDa • Versatile—visualize on a UV transilluminator or laser-based scanning instrument after SDS-PAGE
™
• Storage conditions: upon receipt store at –20°C • Stability: 6 months from date of receipt
Recommended products The BenchMark Fluorescent Protein Standard is recommended for use with NuPAGE gels or Novex Tris-Glycine gels.
BenchMark His-tagged Protein Standard NuPAGE 4–12% Bis-Tris Gel with MES SDS buffer
™
• Versatile—can be visualized with Invitrogen™ InVision™ HisTag In-Gel Stain or Coomassie R-250 stain on SDS-PAGE gels, or with Anti-His (C-term) Antibody using chromogenic or chemiluminescent detection systems
49
SimplyBlue stain
InVision stain
Storage specifications • Storage buffer: Tris-HCl, SDS, glycerol, DTT, and Coomassie Blue G-250 • Storage conditions: upon receipt store at –20°C • Stability: 6 months from date of receipt
Recommended products The BenchMark His-tagged Protein Standard is recommended for use with NuPAGE gels and Novex Tris-Glycine gels.
Learn more at thermofisher.com/specialtystandards
Learn more at thermofisher.com/specialtystandards
IEF Marker 3-10 Accurate determination of protein isoelectric points The IEF Marker 3-10 is a ready-to-use protein standard developed for IEF applications. This marker can be used for monitoring of protein separation on IEF gels and pI determination of unknown protein samples. • Comprehensive—13 purified isoforms from pI 3.5–10.7; no additional high range or low range markers are required • Versatile—can be used for both native and denaturing conditions
IEF Marker 3-10 Novex pH 3–10 Gel
Storage specifications • Storage buffer: 10% glycerol containing bromophenol blue (0.01%) and methyl red (0.01%) • Storage conditions: upon receipt store at –20°C • Stability: 1 year from date of receipt
Learn more at
Recommended products
thermofisher.com/iefstandards
The IEF Marker 3-10 is applicable to all IEF gels (vertical or horizontal).
For ordering information refer to page 86.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
50
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
51
Electrophoresis chambers and power supplies Electrophoresis run considerations: In electrical , the process of electrophoresis is closely associated with the following equations derived from Ohm’s Law: Voltage = Current × Resistance (V = IR) Wattage = Current × Voltage (W = IV)
Which electrophoresis chamber system is right for you?
Resistance
Current
The electrical resistance of the assembled electrophoresis cell is dependent on buffer conductivity, gel thickness, temperature, and the number of gels being run. Although the resistance is determined by the gel system, the resistance varies over the course of the run.
For a given gel/buffer system, at a given temperature, current varies in proportion to the field strength (voltage) and crosssectional area (thickness and number of gels). When using a constant current setting, migration starts slow, and accelerates over time, thus favoring stacking in discontinuous gels.
• In discontinuous buffer systems (and to a lesser extent in continuous buffer systems) resistance increases over the course of electrophoresis. This occurs in the Tris-glycine buffer system as highly conductive chloride ions in the gel are replaced by less conductive glycine ions from the running buffer.
When running under constant current, set a voltage limit on the power supply at, or slightly above the maximum expected voltage to avoid unsafe conditions. At constant current voltage increases as resistance increases. If a local fault condition occurs (e.g., a bad connection), high local resistance may cause the voltage to reach the maximum for the power supply, leading to overheating and damage of the electrophoresis cell.
• Resistance decreases as the temperature increases.
Voltage The velocity of an ion in an electric field varies in proportion to the field strength (volts per unit distance). The higher the voltage, the faster an ion moves. For most applications, we recommend a constant voltage setting. • A constant voltage setting allows the current and power to decrease over the course of electrophoresis, providing a safety margin in case of a break in the system. • The constant voltage setting does not need adjustment to for differences in number or thickness of gels being electrophoresed.
For ordering information refer to page 87.
Mini Gel Tank
XCell SureLock Mini-Cell
XCell4 SureLock Midi-Cell
Gel capacity
Up to 2 minigels
Up to 2 minigels (8 x 8 cm)
Up to 4 midigels (8 x 13 cm)
Cell dimensions (L x W x H)
32 x 11.5 x 16 cm (height with lid on)
14 x 13 x 16 cm (height with lid on)
21 x 19 x 16 cm (height with lid on)
Advantages
• The Mini Gel Tank is versatile and compatible with NuPAGE, Bolt, or Novex minigels. The unique tank design enables convenient sideby-side gel loading and enhanced viewing during use. • Mini Blot Module is available for wet protein transfers.
• XCell II Blot Module is available for semi-wet protein transfers • Instrument incorporates a gel tension wedge in place of the rear wedge used on earlier models
• Advanced apparatus for easier, more reliable electrophoresis with midigels
Power Wattage measures the rate of energy conversion, which is manifested as heat generated by the system. Using constant power ensures that the total amount of heat generated by the system remains constant throughout the run, but results in variable mobility since voltage increases and current decreases over the course of the run. Constant power is typically used when using IEF strips. When using constant power, set the voltage limit slightly above the maximum expected for the run. High local resistance can cause a large amount of heat to be generated over a small distance, damaging the electrophoresis cell and gels.
Learn more at thermofisher.com/electrophoresischambers
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
52
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
53
Mini Gel Tank One tank, 181 gels The Mini Gel Tank is designed for more intuitive use and greater convenience compared to traditional electrophoresis tanks (Figure 24). The unique, side-by-side tank design allows you to perform electrophoresis of 1 or 2 minigels.
1. Snap the electrophoresis tank into the base, and place the cassette clamp(s) into the chamber(s) with the anode connector(s) (+) aligned to the center.
2. Remove the comb, and peel away the tape at the bottom of the gel cassette.
3. Place the cassette in the chamber with the wells facing towards you.
Rinse the wells 3 times with 1X buffer.
Hold the cassette in a raised position and close the clamp by moving the cam handle forward.
Fill the chamber(s) with 1X buffer to the level of the cathode.
The Mini Gel Tank offers: • Versatility—compatible with all of our minigels, including NuPAGE, Novex, Bolt, and specialty gels • Easy sample loading—forward-facing well configuration • Simultaneous visualization of both gels—streamlined, side-by-side tank configuration • Simple monitoring of gels—white tank stand provides easy visualization of prestained markers • Less running buffer required—gel chambers are separated, so you only need to load sufficient buffer for each gel to the specified fill line
Specifications • Gel capacity: up to 2 minigels • Cell size (L x W x H): 32 x 11.5 x 16 cm (height with lid on) • Buffer requirement: 400 mL for each minigel chamber • Material: polycarbonate • Chemical resistance: not compatible with acetone, chlorinated hydrocarbons, or aromatic hydrocarbons
4. Make sure the wells are completely filled with 1X buffer. Load your samples and markers.
5. Hold the cassette and release the cassette clamp.
6. Make sure the power supply is off.
Gently lower the casette so that it rests on the bottom of the chamber, and close the cassette clamp.
If only running one gel, remove the cassette clamp from unused chamber.
Add 1X buffer to the level of the fill line.
Place the lid on the tank and plug the electrode cords into the power supply. Turn the power supply on to begin electrophoresis.
Figure 24. How to use the Mini Gel Tank.
Figure 25. Electrophoresis of Bolt gel using the Mini Gel Tank. Protein standards and samples were loaded at 10 µL sample volumes in an Invitrogen™ Bolt™ 4–12% Bis-Tris Plus Gel. Electrophoresis was performed using the Mini Gel Tank at 200 V (constant). Sharp, straight bands with consistent migration patterns were observed after staining with SimplyBlue SafeStain. Images were acquired using a flatbed scanner. Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 µg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 µg HeLa cell lysate; Lane 5: 20 µg HeLa cell lysate; Lane 6: 5 µg BSA; Lane 7: 40 µg Jurkat cell lysate; Lane 8: 5 µg GST fusion protein; Lane 9: Novex Sharp Unstained Protein Standard; Lane 10: 5 µg β-galactosidase.
Watch our Mini Gel Tank video. thermofisher.com/minigeltank
Recommended products The Mini Blot Module is a wet transfer device that conveniently fits into the chambers of the Mini Gel Tank to easily transfer proteins from minigels to nitrocellulose or PVDF membranes.
Learn more at thermofisher.com/minigeltank For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
54
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
XCell SureLock Mini-Cell
55
Figure 26. How to use the XCell SureLock Mini-Cell.
Simultaneous electrophoresis of up to 2 minigels The unique design of the Invitrogen™ XCell™ SureLock Mini-Cell allows you to run minigels quickly and easily without any clamps or grease (Figure 26). The tight seal provided by the gel tension wedge results in leak-free, consistent performance. The XCell SureLock Mini-Cell is compatible with NuPAGE, Novex, and specialty gels (Figure 27).
1. Drop buffer core into the lower 2. Lock the gel tension wedge in buffer chamber of the XCell place, load samples, and fill the SureLock Mini-Cell. Insert one buffer chambers with the minigel in front of the buffer core and appropriate running buffers. a second minigel or the buffer dam behind the buffer core.
Key features of the XCell SureLock Mini-Cell:
Specifications
• -friendly design—uses single gel tension wedge with no clamps or grease
• Gel capacity: up to 2 minigels
• Flexibility—perform electrophoresis of 2 minigels simultaneously
• Buffer chamber requirement (Novex minigels):
• Unique, heat dissipating design—no need for a cooling device • Built-in safety features—retractable plugs, recessed jacks, and a specially designed lid enhances safety
• Chemical resistance: The XCell SureLock Mini-Cell is impervious to most alcohols but not compatible with acetone, chlorinated hydrocarbons (e.g., chloroform), or aromatic hydrocarbons (e.g., toluene, benzene)
Learn more at thermofisher.com/surelockmini
For ordering information refer to page 87.
Figure 27. Electrophoresis of NuPAGE Bis-Tris gels with the XCell SureLock Mini-Cell. Lane 1: SeeBlue Plus2 Prestained Standard; Lane 2: 10 µg E. coli lysate; Lane 3: Mark12 Unstained Standard (blend of 12 purified proteins); Lane 4: 40 µg HeLa cell lysate; Lane 5: 20 µg HeLa cell lysate; Lane 6: not used; Lane 7: 40 µg Jurkat cell lysate; Lane 8: 5 µg of a GST fusion protein; Lane 9: Invitrogen™ Novex™ Sharp Protein Standard; and Lane 10: 5 µg β-galactosidase. Gel electrophoresis was performed at 200 V (constant) and gels were stained using SimplyBlue SafeStain. Images were acquired using a flatbed scanner.
• Cell size (L x W x H): 14 x 13 x 16 cm (height with lid on)
–– Upper buffer chamber: 200 mL –– Lower buffer chamber: 600 mL
3. Place the cell lid on the unit and you’re ready to run.
NuPAGE Bis-Tris gel in XCell SureLock Mini-Cell
Recommended products The XCell SureLock Mini-Cell can be easily adapted for transfer of proteins from minigels to membranes by simply inserting the XCell II Blot Module in the lower buffer chamber.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
56
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
XCell4 SureLock Midi-Cell
A.
Simultaneous electrophoresis of up to 4 midigels The Invitrogen™ XCell4 SureLock™ Midi-Cell allows simultaneous vertical electrophoresis of 1–4 midigels without leaking, enabling consistent performance. The system is designed to dissipate heat effectively and evenly, and enable highresolution results when using Novex midigels (Figure 29).
B.
1. Insert the XCell4 SureLock Assembly in its unlocked position into the center of the Midi-Cell base. The XCell4 SureLock Assembly slides down over the protrusion in the Midi-Cell base.
4. The upper buffer chamber (cathode) is the void formed between a gel and the buffer core at the center of each core.
2. Place one cassette on each side of the buffer core for each of the two cores. For each cassette, the shorter “well” side of the cassette must face out towards the lower buffer chamber.
5. Lock the XCell4 SureLock Assembly by moving the tension lever to the locked position (indicated on the XCell4 SureLock Assembly). This will squeeze the gels and buffer cores together, creating leak-free seals.
3. While holding the assembly together with your hands (A), insert the buffer cores with the gel cassettes into the lower buffer chamber such that the negative electrode fits into the opening in the gold plate on the lower buffer chamber (B). Always hold the cassette assembly by its edges as shown in the figure.
6. Proceed to loading samples and buffers.
Note: If you are having difficulty inserting the assembly into the lower buffer chamber, make sure the cathode (black polarity indicator) of the buffer core is aligned with the cathode (black polarity indicator) of the lower buffer chamber.
Specifications Key features of the XCell4 SureLock Midi-Cell: • -friendly design—leak-free electrophoresis without clamps or grease • Flexibility—perform electrophoresis of 1–4 midigels • Unique, heat dissipating design—no need for a cooling device • Built-in safety features—specially designed lid enhances safety
57
• Gel capacity: up to 4 midigels (8 x 13 cm) • Cell size (L x W x H): 21 x 19 x 16 cm (height with lid on) • Buffer chamber requirement: –– Upper buffer chamber: 175 mL x 4 –– Lower buffer chamber: 540–700 mL • Chemical resistance: not compatible with acetone, chlorinated hydrocarbons, or aromatic hydrocarbons
Figure 28. How to use the XCell4 SureLock Midi-Cell with 4 gels.
1
2
3
4
5
6
7
8
9
10 11
12
13
14
15
16
17
18
19 20
Figure 29. Quality of a precast NuPAGE Novex 4–12% Bis-Tris Midi Gel with a variety of protein standards, lysates and purified proteins. Electrophoresis was performed using MES running buffer and an XCell4 SureLock Midi Cell at 200 V (constant). Following electrophoresis, the gel was stained using SimplyBlue SafeStain, destained using water, and imaged using a flatbed scanner. Sharp, straight bands were observed. Lanes 1, 10, 11, and 20 were each loaded with 5 µL of Mark12 Unstained Standard (blend of 12 purified proteins). Lanes 2, 9, 12, and 19 were each loaded with 10 µg of E. coli lysate. Lanes 3 and 18 were each loaded with 6 µg of human IgG. Lanes 4 and 17 were each loaded with 6 µg of human IgM. Lanes 5 and 16 were each loaded with 5 µL of SeeBlue Plus2 Prestained Protein Standard. Lanes 6 and 15 were each loaded with 5 µL of BenchMark Protein Ladder. Lanes 7 and 14 were each loaded with 15 µL of MagicMark XP Western Protein Standard. Lanes 8 and 13 were each loaded with 5 µL of HiMark Unstained Protein Standard.
Learn more at thermofisher.com/surelockmidi For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
58
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Post stain
Select precast gel
Prepare samples and select buffers
Choose the electrophoresis chamber system and power supply
Select the standard
Stain the gel
Run the gel
Post stain
59
Run the gel PowerEase 90W Power Supply
Table 4. Gel running conditions in electrophoresis chamber systems. Running conditions in XCell Surelock Mini-Cell
Simple, affordable power supply specifically for minigel electrophoresis
Bolt 4–12% (MES)
The Invitrogen™ PowerEase™ 90W Power Supply is designed specifically for minigel electrophoresis. The straightforward, intuitive interface makes the powering of gel runs a simple and easy process. In addition, the PowerEase 90W Power Supply features: • Constant voltage or current settings • Built-in timer for walk-away gel electrophoresis • Output jacks that are compatible with most electrophoresis devices
PowerEase 300W Power Supply
Voltage (V)
Starting current (mA)*
End current (mA)*
Approximate run time (minutes)
NA
NA
NA
NA
Running conditions in Mini Gel Tank
Voltage (V)
Starting current (mA)*
Approximate End current run time (mA)* (minutes)
200
160
70
20
Bolt 4–12% (MOPS)
NA
NA
NA
NA
200
160
50
35
NuPAGE 4–12% Bis-Tris (MES)
200
100 to 125
60 to 80
35
200
160
90
30
NuPAGE 4–12% Bis-Tris (MOPS)
200
100 to 125
60 to 80
50
200
140
50
42
Novex 4–20% Tris-Glycine (denatured)
125
30 to 40
8 to 12
90
125
40
10
100
Novex 4–20% Tris-Glycine (native)
125
6 to 12
3 to 6
1 to 12 hours
125
30
10
90
NuPAGE 3–8% Tris-Acetate (denatured)
150
40 to 55
25 to 40
60
150
60
20
50
NuPAGE 3–8% Tris-Acetate (native)
150
18
7
2 to 3 hours
150
40
10
100
Novex 10–20% Tricine
125
80
40
90
125
110
40
65
NativePAGE 3–12%
150
12 to 16
2 to 4
90 to 115
150
10
<10
80
pH 3-10 IEF
10% Zymogram (Gelatin)
100
7
NA
60
100
8
NA
60
200
NA
NA
60
200
NA
NA
60
500
NA
5
30
500
NA
5
30
125
30 to 40
8 to 12
90
125
40
10
90
* Per gel. Note: Run times may vary depending on the power supply and gel percentage.
Learn more at thermofisher.com/powerease
Programmable power supply designed for high-throughput gel electrophoresis The Invitrogen™ PowerEase™ 300W Power Supply is a fully programmable power supply designed for high-throughput gel electrophoresis. The straightforward, intuitive interface makes the powering of gel runs a simple and easy process. In addition, the PowerEase 300W Power Supply features: • Constant voltage, current, or power settings • Built-in timer for walk-away gel electrophoresis • Up to 10 custom programs with 10 steps each • Four sets of output jacks that are compatible with most electrophoresis devices
For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
60
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
61
Troubleshooting tips XCell SureLock Mini-Cell troubleshooting
Electrophoresis troubleshooting
Observation
Cause
Solution
Problem
Possible cause
Suggested solution
Run taking longer than usual
Buffers are too dilute
Check if buffer was diluted properly. Check buffer recipe; dilute from concentrate or remake if necessary.
Run taking longer time with recommended voltage
Running buffer too dilute
Make fresh running buffer and use a 1X dilution.
Upper buffer chamber is leaking
Make sure the buffer core is firmly seated, the gaskets are in place and the gel tension lever is locked.
Running buffer too concentrated
Make fresh running buffer and use a 1X dilution.
Voltage is set too low
Set correct voltage.
Current too high and excessive heat generated with recommended voltage
Remove tape from bottom of cassette.
Current too low or no current with recommended voltage
Incomplete circuit
Tape left on the bottom of the cassette Connection to power supply not complete
Check all connections with a voltmeter for conductance.
Remove the tape from the bottom of the gel cassette prior to electrophoresis. Make sure the buffer covers sample wells; check the wire connections on the buffer core.
Insufficient buffer level
Make sure the upper buffer (cathode) is covering the wells of the gel. Be sure there is sufficient buffer in the Lower Buffer Chamber to cover the slot at the bottom of the gel.
Streaking of proteins
Sample overload
Load less protein.
High salt concentration in sample
Decrease the sample salt concentration by dialysis or gel filtration.
Sample precipitates
Increase the concentration of SDS in the sample.
Contaminants such as lipids or DNA complexes in sample
Centrifuge or clarify the sample to remove particulate contaminants. Treat sample with nuclease(s).
Poorly poured gel
Make sure the gel is poured evenly and all at once.
Protein sample only partially denatured
Fully denature the protein.
Protein sample only partially reduced
Make sure a sufficient amount of DTT or β-mercaptoethanol is added.
Gel runs for too long
Watch the dye front as an indicator for proper running time.
Loading a large volume of sample causes incomplete stacking
Load appropriate volume of sample. If the sample is too dilute, concentrate it using ultrafiltration.
Uneven electric field during run
Try to make sure the loading is symmetrical if the protein concentration is known.
Uneven surface of the resolving gel
Try to make the resolving gel surface even while pouring the gel.
Expired gels
Use the gels before the specified expiration date; Note: NuPAGE gels have an extended 12 month shelf life, minimizing the risk of having expired gels.
Current reading on power supply is zero or very low
Run is faster than normal with Buffers are too concentrated poor resolution or incorrect
Cannot see the sample wells to load sample
Check buffer recipe; dilute or re-make if necessary.
Voltage, current, or wattage is set at a higher limit
Decrease power conditions to recommended running conditions (see page 59).
There is little contrast between the sample well and the rest of the gel
Mark cassette at the bottom of the wells with a marker pen prior to assembling the Upper Buffer Chamber. Illuminate the bench area with a light source placed directly behind the XCell SureLock unit.
Fuzzy bands
Mini Gel Tank troubleshooting Observation
Cause
Solution
Run taking longer than usual
Buffers are too dilute
Check buffer recipe; dilute from concentrate or remake if necessary.
Buffer chamber is leaking
Make sure the cassette clamp is firmly seated, the gaskets are in place and the cassette clamp is locked.
Current is set too low
Set correct current.
Tape left on the bottom of the cassette
Remove tape from bottom of cassette.
Connection to power supply not complete
Check all connections with a voltmeter for conductance.
Insufficient buffer level
Make sure there is sufficient buffer in the electrophoresis tank to cover the wells of the gel.
Current reading on power supply is zero or very low
Run is faster than normal with Buffers are too concentrated poor resolution or incorrect
Cannot see the sample wells to load sample
Dumbbell shaped bands or “smiling” bands
Check buffer recipe; dilute or re-make if necessary.
Current is set at a higher limit
Decrease current to recommended running conditions (see page 59).
There is little contrast between the sample well and the rest of the gel
Mark cassette at the bottom of the wells with a marker pen prior to placing the cassette in the electrophoresis tank.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
62
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Stain the gel
Run the gel
Stain the gel
Protein stains
Silver stains
Once protein bands have been separated by electrophoresis, they can be directly visualized using different methods of in-gel detection. Over the past several decades, demands for improved sensitivity for small sample sizes and compatibility with downstream applications and detection instrumentation have driven the development of several basic staining methods. Each method has particular advantages and disadvantages, and a number of specific formulations of each type of method provide optimal performance for various situations.
• Invitrogen™ SilverXpress™ Silver Stain
Protein gel electrophoresis technical handbook
Post stain
Coomassie dye protein gel stains
• Thermo Scientific™ Pierce™ Silver Stain
• Thermo Scientific™ Pierce™ Silver Stain for Mass Spectrometry
Fluorescent/specialty stains
Coomassie stains
• Pierce Reversible stain for nitrocellulose or PVDF membranes • Pro-Q™ Emerald Glycoprotein stain • Pro-Q™ Diamond Phosphoprotein stain To visualize the proteins, a protein-specific, dye-binding or color-producing chemical reaction must be performed on the
™
• SimplyBlue SafeStain • Thermo Scientific™ Imperial™ Protein Stain
The most common methods for in-gel protein detection use stains with Coomassie dye. These stains use either the G-250 (colloidal) or R-250 form of the dye (Table 6). Colloidal Coomassie stain can be formulated to effectively stain proteins within one hour and require only water (no methanol or acetic acid) for destaining.
of the stain, various steps are necessary to hold the proteins in the matrix and to facilitate the necessary chemical reaction. Most staining methods involve some version of the same general incubation steps: S
DI H2O
DI H2O
shown for SimplyBlue SafeStain (Figure 30, 33 and 34), PageBlue (Figure 31 and 35).
• Simple—Coomassie dye–based formulations are easy to formulate and are widely used
Learn more at thermofisher.com/coomassiestains
• Economical—Coomassie dye–based stain formulations are cost effective 1. Water wash.
2. Fix.
3. Water wash.
4. Stain.
5. Destain.
• A water-wash to remove electrophoresis buffers from the gel matrix • An acid or alcohol wash to condition or fix the gel to limit diffusion of protein bands from the matrix
• Flexible—useful for qualitative visualization, quantitative densitometry, and gel excision and analysis by mass spectrometry Table 6. Coomassie dye–based protein gel stains. SimplyBlue SafeStain
Imperial Protein Stain
PageBlue Protein Staining Solution
Type
G-250
R-250
G-250
Limit of detection
>7 ng
3 ng
5 ng
Time to stain (min)
12
60
60
Depending on the particular staining method, two or more of
Compatible with: PVDF membranes Nitrocellulose membranes
Yes No
Yes No
Yes No
these functions can be accomplished with one step. For example,
Reusable
No
No
Yes (up to 3x)
fix and stain in one step. Conversely, certain functions require
Mass spectrometry compatible
Yes
Yes
Yes
several steps. For example, silver staining requires both a
Color
Purple
Purple
Blue-green
Feature
Free of methanol and acetic acid
Photographs better than Coomassie G-250 dye
Free of methanol and acetic acid
Advantages
Rapid, sensitive completely non-hazardous (does not require methanol or acetic acid fixatives or destains) staining
Fast, ultrasensitive protein detection
Cost-effective option for fast, sensitive staining
• Treatment with the stain reagent to allow the dye or chemical to diffuse into the gel and bind (or react with) the proteins • Destaining to remove excess dye from the background gel matrix
staining reagent step and a developer step to produce the colored reaction product.
Learn more at thermofisher.com/proteinstains For ordering information refer to page 87.
with simplified protocols. Example data and staining protocols are
Key features:
• Easy to use—simply soak the gel in stain solution and destain to observe protein bands
S
Our Coomassie stains provide sensitive protein detection along
Protein Staining Solution (Figure 32), and Imperial Protein Stain
proteins within the gel. Depending on the particular chemistry
a dye reagent that is formulated in an acidic buffer can effectively
• Thermo Scientific PageBlue stain ™
Convenient, ready-to-use reagents with no permanent chemical modification
• Invitrogen™ SYPRO™ Orange, Red, or Ruby gel stain
Typically these stains can be classified broadly based on the dye or molecule that helps visualize the protein stains:
63
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
64
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
Protocols
65
Example data 1 2 3 4 5 6 7 8 9 10 2
3
1
DI H2O
S
DI H2O
DI H2O
Figure 30. SimplyBlue SafeStain protocol.
1. Wash the gel three times (5 minutes) with ultrapure water.
2. Add SimplyBlue SafeStain (1 hour).
3. Wash gel with 100 mL of DI water for 1 hour.
Figure 33. Sensitive staining results with SimplyBlue SafeStain. The following samples were separated on a NuPAGE Novex 4-12% Bis-Tris gel and then stained with SimplyBlue SafeStain. Lane 1: 6 µg protein mix; Lane 2: 1 µg rabbit IgG; Lane 3: 1 µg reduced BSA; Lane 4: 5 µg E. coli lysate; Lane 5: 20 ng reduced BSA; Lane 6: 10 ng reduced BSA; Lane 7: 7 ng reduced BSA; Lane 8: 3 ng reduced BSA; Lane 9: 10 µL Mark12 Unstained Standard (blend of 12 purified proteins); Lane 10: 5 µL Mark12 Unstained Standard.
4. Additional water wash with 100 mL of DI water (1 hour) for increased sensitivity.
1
3 4
5
6
7
8
9
1
2
3 4
5
6
7
8
9
1
2
3 4
5
6
7
8
9
Rabbit IgG
S
DI H2O
2
Figure 34. Two-dimensional electrophoresis (2DE) analysis of spinach chloroplast extract; staining with SimplyBlue SafeStain. Spinach chloroplast extract was prefractionated in the ZOOM™ IEF Fractionator and the individual fractions were then separated by 2DE using narrow pH range ZOOM™ Strips and NuPAGE™ Novex 4–12% Bis-Tris ZOOM™ Gels. Gels were Coomassie stained using SimplyBlue SafeStain.
DI H2O BSA Protein A
Figure 31. Imperial Protein Stain protocol.
1. Wash the gel three times with deionized water (15 minutes).
2. Add Imperial Protein Stain (5 minutes−1 hour).
3. Water destain (15 minutes−overnight).
Protein G Lysozyme
5-minute stain; 15-minute water destain
1-hour stain; 2-hour water destain
1-hour stain; overnight water destain
Figure 35. Enhanced sensitivity and clear background using Imperial Protein Stain. For even greater sensitivity and reduced background, gels can be stained with Imperial Protein Stain for 1 hour and washed with water from 1 hour to overnight. Lane 1: BSA only (6 µg); Lane 2–9: loaded left to right with 1,000, 200, 100, 50, 25, 12, 6, and 3 ng protein sample.
Did you know? DI H2O
S
DI H2O
Staining with a Coomassie stain prior to silver staining allows for more uniform staining of certain proteins since silver ions can interact with certain functional groups such as carboxylic acid groups, imidazole, sulfhydryls, and amines.
DI H2O
Recommended products
1. Wash the gel three times with ultrapure water (30 minutes).
2. Add PageBlue Protein Staining Solution (1 hour).
For ordering information refer to page 87.
3. Rinse gel two times with ultrapure water (<1 minute)
4. Wash gel one time with ultrapure water (5 minutes)
Figure 32. PageBlue Protein Staining Solution protocol.
The Thermo Scientific Pierce Power Stainer is designed for rapid Coomassie dye staining of proteins in up to two minigels and subsequent removal of unbound stain from the gel in a single step. Refer to page 72 of this brochure for more information.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
66
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Silver stains
We offer highly sensitive silver stains with short protocol times that are also compatible with mass spectrometry (Table 7). The SilverXpress™ Silver Staining Kit provides nanogram-level sensitivity
Pierce Silver Stain for Mass Spectrometry
Pierce Silver Stain Kit
SilverXpress Silver Staining Kit
Components (steps)
6 (17)
4 (15)
5 (13)
Time required
1 hr 13 min
2 hr 25 min
2 hr
Limit of detection
0.25 ng
0.25 ng
0.86 ng
Mass spectrometry compatible
Yes
Yes
Yes
Storage
Room temperature
Room temperature
4°C
Stability
1 year
1 year
6 months
Advantages
• Fast and sensitive staining and destaining of protein gels • Optimized for peptide recovery after in-gel typsin digestion for mass spectrometry • Flexible gel fixation (15–30 min to overnight) and staining (1–30 min)
• Rapid, ultrasensitive and versatile silver stain system • Flexible gel fixation (30 min to overnight) and staining (5 min to 20 hours)
• Nanogram-level sensitivity for silver staining with minimal background
Protocols and example data
with minimal background (Figure 37), while the Pierce™ Silver Stain Kit provides protocol flexibility (Figure 38 and 39).
SZ
F
H2O
1 2 3 4 5 6 7 8 9 10
Key features: • Sensitive—silver stains are highly sensitive stains that allow for visualization of proteins at sub-nanogram levels
Learn more at thermofisher.com/silverstains 1. Wash the gel with water.
• Easy to use and flexible—optimized for minimal steps and flexibility to accommodate shorter or longer protocols • Workflow compatible—our mild chemical formulations help ensure compatibility with mass spectrometry and sequencing • Robust performance—detailed protocol enables consistent results with clear background
67
Table 7. Silver stain kits.
Ultra-sensitive stains with optimized protocols, manufactured for minimal variability Silver staining is the most sensitive colorimetric method for detecting total protein, and functions by the deposition of metallic silver at the location of protein bands. Silver ions (from silver nitrate in the stain reagent) interact and bind with certain protein functional groups. The strongest interactions occur with carboxylic acid groups (Asp and Glu), imidazole (His), sulfhydryl groups (Cys), and amines (Lys). Various sensitizer and enhancer reagents are essential for controlling the specificity and efficiency of silver ion binding to proteins and effective conversion (development) of the bound silver to metallic silver.
Protein gel electrophoresis technical handbook
Post stain
2. Fix the gel in Fixing Solution for 10 minutes.
3. Decant the Fixing Solution and incubate the gel in 2 changes of Sensitizing Solution. S
H2 O S 4. Decant the Sensitizing Solution and rinse the gel two times with ultrapure water.
5. Incubate the gel in Staining Solution.
D
H2O
D 6. Decant the Staining Solution and rinse the gel two times with ultrapure water.
7. Incubate the gel in Developing Solution.
SS
Figure 37. Crystal clear background with the SilverXpress Kit. Samples were separated on a NuPAGE Novex 4-12% Bis-Tris gel and stained with the SilverXpress Kit. Lanes 1, 10: Mark12 Unstained Standard (blend of 12 purified proteins) diluted 1:4; Lane 2: Mark12 Unstained Standard diluted 1:8; Lane 3: Mark12 Unstained Standard diluted 1:16; Lane 4: Mark12 Unstained Standard diluted 1:32; Lane 5: Mark12 Unstained Standard diluted 1:64; Lane 6: 1.6 ng BSA; Lane 7: 0.8 ng BSA; Lane 8: E. coli lysate diluted 1:20; Lane 9: E. coli lysate diluted 1:80.
H 2O
8. Add the Stopping Solution directly to the gel when the desired staining intensity is reached.
9. Decant the Stopping Solution and wash the gel three times with ultrapure water.
Figure 36. SilverXpress Silver Staining Kit protocol.
For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
68
Prepare samples and select buffers
Select precast gel
Select the standard
Acetic Acid
EtOH H2O
Choose the electrophoresis chamber system and power supply
Run the gel
Lysate staining
Stain the gel
MW marker staining
F
2. Fix 2 x 15 minutes in EtOH/ acetic acid.
SZ
10% EtOH
SZ 3. Incubate 2 x 5 minutes with 10% EtOH. Wash.
4. Mix Sensitizer. Sensitize for 1 minute. Wash 2 x 1 minute.
Fluorescent gel stains are designed for use in 1D and 2D PAGE and offer sensitivities similar to that obtained with silver staining techniques. Invitrogen™ SYPRO™ protein stains are easy-to-use fluorescent stains for the detection of proteins separated by PAGE (Table 8). Stained proteins can be viewed with a standard UV or blue-light transilluminator or with a laser scanner.
thermofisher.com/fluorescentstains Recommended products For optimal sensitivity with Polaroid™ film, SYPRO™ Photographic Filter is recommended.
• Simple—no destaining or timed steps required; minimal hands-on time
5. Mix Silver Stain. Stain for 5 minutes. Wash 2 x 20 seconds.
5% Acetic Acid
• Quantitative—linear quantitation range over two orders of magnitude with low protein-to-protein variability • Highly sensitive—typically more sensitive than Coomassie dye–based stains and equivalent to silver stains
D 7. Remove developer. Stop with 5% Acetic Acid for 10 minutes.
Table 8. SYPRO protein stains.
Figure 38. Pierce Silver Stain Kit protocol.
For ordering information refer to page 87.
Learn more at
Features:
S
6. Mix Developer. Develop for 2-3 minutes.
2 minutes, 30 seconds
Development Time
Figure 39. Pierce Silver Stain Kit exhibits excellent senstivity. In standard minigels, proteins are detectable at greater than 0.25 ng per band or spot.
S
D
2 minutes, 30 seconds
69
Fluorescent protein gel stains Rapid, highly sensitive fluorescent stains for total protein detection after electrophoresis
F 1. Wash 2 x 5 minutes with ultrapure water.
Protein gel electrophoresis technical handbook
Post stain
SYPRO Ruby stain
SYPRO Orange stain
SYPRO Red stain
Limit of detection
0.25 ng
4–8 ng
4–8 ng
Stain and destain time
90 min microwave; 18 hr standard
~1 hr
~1 hr
Ex/Em
280 nm, 450/610 nm
300 nm, 470/510 nm
300 nm, 550/630 nm
Ease of use
Ready to use
Supplied as stock solution
Supplied as stock solution
Compatible applications
Mass spectrometry, IEF, 2D gels, on-membrane staining
Mass spectrometry, IEF, 2D gels, on-membrane staining
Mass spectrometry, IEF, 2D gels, on-membrane staining
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
70
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
71
Stain the gel
Specialty protein stains
Electrophoretic staining technology—Pierce Power Stainer
Our specialty protein stains include in-gel phosphoprotein and glycoprotein detection and on-membrane reversible protein staining kits (Table 9).
Learn more at thermofisher.com/specialtystains
Table 9. Specialty protein stains. Pro-Q Emerald 488 Glycoprotein Gel and Blot Stain Kit
Pro-Q Emerald 300 Glycoprotein Gel and Blot Stain Kit
Pro-Q Diamond Phosphoprotein Gel Staining Kit
Detects
Glycoproteins
Glycoproteins
Phosphoproteins
Sensitivity
4 ng glycoprotein per band
0.5 ng glycoprotein per band
1–16 ng phosphoprotein per band
Stain and destain time
~6 hr
~5 hr
4–5 hr
Ex/Em
510/520 nm
280/530 nm
555/580 nm
Advantages
Selective staining of glycoproteins
Selective staining of glycoproteins
Selective staining of phosphoproteins
The Thermo Scientific Pierce Power Stainer consists of a Thermo Scientific™ Pierce™ Power Station with activated Staining Software and a Thermo Scientific™ Pierce™ Power Stain Cassette. It is designed for rapid Coomassie staining and destaining of proteins in polyacrylamide gels. Traditional Coomassie staining techniques require one hour to overnight staining and destaining to achieve desired results. When used in conjunction with Thermo Scientific™ Pierce™ Midi and Mini Gel Power Staining Kits, the Pierce Power Stainer is designed to provide staining efficiency in as few as 6 minutes that is equivalent to, or better than, traditional Coomassie staining techniques.
Good to know
How does electrostaining work? Cathode (–)
Coomassie staining pad Pre-run and pre-washed SDS-PAGE gel Destaining pad
Anode (+)
Pierce Power Stain Cassette Cathode (–) Staining pad Gel Destaining pad Anode (+)
The significant reduction in protein staining time is accomplished by utilizing inonic Power Stain Solution and Destain Solution to electrophoretically drive the negatively charged Coomassie R-250 dye out of the top gel pad, through the polyacrylamide gel matrix and the bottom gel pad, and toward the positively charged anode.
Watch our Pierce Power Stainer video. thermofisher.com/powerstainer
For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
72
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook
Post stain
73
Pierce Power Stainer Rapid Coomassie dye staining and destaining in approximately 10 minutes The Thermo Scientific™ Pierce™ Power Stainer is designed for rapid Coomassie dye staining of proteins in polyacrylamide gels and subsequent removal of unbound stains to give sharply stained protein bands with minimal or no background. The Pierce Power Stainer offers:
Specifications
• Speed—Coomassie dye staining and destaining of proteins in about 10 minutes
• Mode of transfer: semi-dry blotting
• Convenience—simultaneously stain and destain 1–2 minigels or 1 midigel • Reliable performance—enables staining results that are equivalent to traditional staining techniques • Easy touch programming—intuitive LCD touch-screen interface includes preprogrammed protocols
Recommended products
• Gel compatibility: SDS-PAGE gels
The Pierce Power System can be used both for fast Coomassie dye staining of protein gels and for rapid semi-dry transfer of proteins from gel to membrane. The Pierce Power Stainer can be upgraded by adding the Pierce™ Power Blot Cassette to make a fully functional Pierce Power System with blotting and staining capabilities.
• Running dimension: horizontal • Platform: Pierce™ Power System
Pierce Power Stainer
Conventional Coomassie stain
Did you know? Conventional Coomassie dye–based staining techniques require 1 hour to overnight incubation.
Total time: 11 minutes 1. Wash gel 1 × 5 minutes in water 2. Power Stain/Destain gel, 6 minutes
Total time: 230 minutes to overnight 1. Wash gel 3 × 10 minutes in water 2. Incubate gel in Coomassie stain solution* for 60 minutes 3. Wash gel 2 × 10 minutes in water 4. Destain gel in destaining solution** for 3 × 20 minutes 5. Incubate gel in water for 60 minutes to overnight
Thermo Scientific Pierce Power Stainer
Thermo Scientific Pierce Power Blotter
*Coomassie stain solution: 45% methanol, 10% acetic acid, 0.25% Coomassie R-250 **Destain solution: 30% ethanol, 5% acetic acid
Figure 40. Pierce Power Stainer saves time and maintains sensitivity.
Learn more at thermofisher.com/powerstainer For ordering information refer to page 87.
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
74
Select precast gel
Prepare samples and select buffers
Select the standard
Choose the electrophoresis chamber system and power supply
Run the gel
Stain the gel
Protein gel electrophoresis technical handbook 75 75
Post stain
Western blotting Transfer and Detection
After electrophoresis, the separated proteins are transferred or blotted onto a second matrix, generally a nitrocellulose or polyvinylidene difluoride (PVDF) membrane. Next, the membrane is blocked to minimize potential nonspecific binding of antibodies to the surface of the membrane. Detailed procedures vary widely for the detection steps of the western blot workflow. One common variation involves direct vs. indirect detection methods. In both the direct and indirect detection methods, the blocked membrane is probed with an antibody (primary antibody) specific to the protein of interest (antigen). In direct detection techniques, this antibody is enzyme conjugated or labeled with a fluorophore. However, in indirect detection techniques, the blocked membrane is probed first with an antibody (primary antibody) which is specific to the antigen followed by another antibody (secondary antibody) raised against the host species of the primary antibody. This secondary antibody is often enzyme conjugated or labeled with a fluorophore. The direct method is not widely used as most researchers prefer the indirect detection method for a variety of reasons.
Horseradish peroxidase (HRP) or alkaline phosphatase (AP) are the most popular enzymes conjugated to antibodies used in the western blot workflow. After incubating the membrane with the detection antibody or antibodies, if an enzyme-conjugated antibody was utilized, an appropriate substrate (chromogenic or chemiluminescent) is added and that results in a detectable product. A popular substrate of choice is a chemiluminescent substrate that, when combined with the enzyme, produces light as a byproduct. With the chemiluminescent substrate, the light output can be captured on film or CCD camera. In recent years fluorescent detection became a popular alternative to the enzymatic detection since it allows for more quantitative data analysis. Fluorescent detection utilizes dye-labeled primary antibodies or dyelabeled secondary antibodies and the signal output is captured on an appropriate imaging system. Whatever substrate is used, the intensity of the signal should correlate with the abundance of the antigen on the blotting membrane.
Key products for western blot transfer: Wet
Semi-dry
Dry
Mini Blot Module
Thermo Scientific Pierce Power Blotter
iBlot™ 2 Dry Blotting System
Key products for western blot detection include: Automated detection
Manual detection Blocking buffers Wash buffers Detergents Enhancers Substrates Stripping buffers X-ray film
We offer a wide range of reagents, kits, equipment, and antibodies to facilitate every step of western blot analysis. iBind™ Flex Western Device
Learn more at thermofisher.com/western
Precast protein gels
Sample preparation and electrophoresis buffers
Protein standards
Electrophoresis chamber systems and power supplies
Electrophoresis run conditions
Protein gel stains
76
Protein gel electrophoresis technical handbook
Appendix
77
Protocol quick reference QUICK REFERENCE
QUICK REFERENCE
NuPAGE Bis-Tris Midi Gels
Bolt™ Mini Gels
Bolt™ Mini Gels
Instructions for performing electrophoresis using Bolt ™ Mini Gels are described below.
Prepare gel 1. Cut open the gel cassette pouch and remove the cassette. 2. Remove the gel comb and rinse wells 3 times with 1X Running Buffer. and tank
Prepare samples
Reagent
3. Remove the tape covering the slot at the lower portion of the cassette.
Reduced Sample Non-reduced Sample
Sample Bolt ™ LDS Sample Buffer (4X) Bolt ™ Reducing Agent (10X) Deionized Water
x µL 10 µL 4 µL to 26 µL
x µL 10 µL — to 30 µL
Load samples
Total Volume 40 µL* 40 µL* Heat samples at 70˚C for 10 minutes. * Scale samples up or down by adjusting all volumes proportionally.
Prepare 1X Buffer Run conditions
Each chamber of the tank requires 400 mL of 1X SDS Running Buffer (mix 20 mL of 20X Bolt ™ MES or MOPS SDS Running Buffer with 380 mL of deionized water). The same buffer type must be used for both chambers.
1–4
Run Bolt™ Mini Gels at constant voltage (1 or 2 mini gels). Running Buffer
Standard Run
Run Time*
MES
200 V
22 min
MOPS
200 V
32 min
Prepare Samples
5–7
Cathode
QUICK REFERENCE
NuPAGE Tris-Acetate Mini Gels
NuPAGE Bis-Tris Mini Gels Reduced Sampl e
Sam pl e NuPAGE LDS Sample Buffer (4X) NuPAGE Reducing Agent (10X) Deionized Water Tot al Vol u m e
x μL 2.5 μL 1 μL to 6.5 μL 10 μ L
Non- r educed Sampl e
Load Buffer
Run
10 μ L
Conditions
Add 50 mL 20X NuPAGE MES or MOPS SDS Running Buffer to 950 mL deionized water to prepare 1X SDS Running Buffer. Load the appropriate concentration of your protein sample on the gel. Fill the Upper (200 mL) and Lower (600 mL) Buffer Chambers with the appropriate 1X Running Buffer. For reduced samples, use 200 mL 1X Running Buffer with 500 μL NuPAGE Antioxidant in the Upper Buffer Chamber. Voltage: Run Time: Expected Current:
Prepare Samples
x μL 2.5 μL -to 7.5 μL
Heat samples at 70˚C for 10 minutes.
Load Sample
Denaturing Sample x µL 2.5 µL — 1 µL to 10 µL final
Native Sample x µL — 5 µL — to 10 µL final
*For reduced samples only.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Add Buffer
Fill Upper Buffer Chamber with 175 mL 1X NuPAGE SDS Running Buffer. For reduced samples, use 175 mL 1X NuPAGE SDS Running Buffer with 435 μL NuPAGE Antioxidant in the Upper Buffer Chamber. Add a sufficient volume of 1X NuPAGE SDS Running Buffer to the Lower Buffer Chamber.
Run
Voltage: Run Time: Expected Current:
200 V constant 40 min (MES Buffer), 55 min (MOPS Buffer) 160–200 mA/gel (start); 120–170 mA/gel (end)
Prepare 1X Buffer
Denaturing samples: Add 50 mL 20X NuPAGE Tris-Acetate SDS Running Buffer to 950 mL deionized water. Native samples: Add 100 mL 10X Tris-Glycine Native Running Buffer to 900 mL deionized water.
Load Sample Load the appropriate concentration of your protein sample on the gel. Add Buffer
Fill Upper Buffer Chamber with 175 mL of the appropriate 1X Running Buffer. For reduced samples, use 175 mL 1X Running Buffer with 435 μL NuPAGE Antioxidant in the Upper Buffer Chamber. Add a sufficient volume of Running Buffer the Lower Buffer Chamber.
Run Conditions
Voltage: Run Time: Expected Current:
150 V constant 70 min (denaturing gel), 2–3 hours (native gel) 70–90 mA/gel (start); 50–60 mA/gel (end); (denaturing gel) 40–45 mA/gel (start); 15–20 mA/gel (end); (native gel)
QUICK REFERENCE
Instructions for electrophoresis using the XCell SureLock Mini-Cell are described below.
Prepare 1X Buffer
Sample NuPAGE LDS Sample Buffer (4X) Tris-Glycine Native Sample Buffer (2X) NuPAGE Reducing Agent (10X)* Deionized Water
Heat denaturing samples at 70˚C for 10 minutes. Do not heat native samples.
Add 50 mL 20X NuPAGE MES or MOPS SDS Running Buffer to 950 mL deionized water to prepare 1X SDS Running Buffer.
Conditions
Reagent
For Research Use Only. Not for use in diagnostic procedures.
For research use only. Not for use in diagnostic procedures.
Reagent
x µL 2.5 µL 1 µL to 10 µL final
Non-reduced sample x µL 2.5 µL — to 10 µL final
Prepare 1X Buffer
* Run times may vary depending upon gel type and power supply.
Prepare Samples
Reduced sample
Prepare Samples
Heat samples at 70˚C for 10 minutes.
Fill Line
Cathode
Reagent Sample NuPAGE LDS Sample Buffer (4X) NuPAGE Reducing Agent (10X) Deionized Water
1. Pre-fill the chamber with 1X Running Buffer to the level of the cathode. 2. Place the cassette in the chamber with the wells facing towards you. Hold the cassette in a raised position and close the cassette clamp. 3. Fill all wells with 1X Running Buffer. 4. Load your samples and markers. 5. Hold the cassette and release the cassette clamp. 6. Gently lower the cassette to the bottom of the chamber, and close the cassette clamp 7. Add 1X buffer to the level of the fill line.
NuPAGE Tris-Acetate Midi Gels
Instructions for electrophoresis of Bis-Tris Gels using the XCell4 SureLock Midi-Cell are described below.
200 V constant 35 minutes (MES Buffer), 50 minutes (MOPS Buffer) 100–125 mA/gel (start); 60–80 mA/gel (end)
Intended Use: For research use only. Not for human or animal therapeutic or diagnostic use.
Prepare 1X Buffer
Reagent Denatur i ng Sampl e* Nati ve Sampl e Sam pl e x μL x μL NuPAGE LDS Sample Buffer (4X) 2.5 μL -Tris-Glycine Native Sample Buffer (2X) -5 μL D ei on i z ed Water t o 7.5 μ L to 5 μL Tot al Vol u m e 10 μ L 10 μ L Samples Heat samples at 70˚C for 10 minutes Do not heat *For reduced samples, add NuPAGE Reducing Agent (10X) to 1X.
Novex Tris-Glycine Mini Gels
Novex Tris-Glycine Mini Gels
Instructions are provided below for electrophoresis of NovexTris-Glycine Gels using the XCellSureLock Mini-Cell.
Non-Denaturing (Native) Electrophoresis
Denaturing Electrophoresis
Prepare Samples
Reagent Sampl e Sample x μL Tris-Glycine Native Sample Buffer (2X) 5 μL Deionized Water to 5 μL Total Volume 10 μL Do not heat samples for native electrophoresis.
Prepare 1X Buffer
Add 100 mL 10X Tris-Glycine Native Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine Native Running Buffer.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Load Buffer
Fill the Upper Buffer Chamber with 200 mL and the Lower Buffer Chamber with 600 mL of 1X Tris-Glycine Native Running Buffer.
Run
Voltage: Run Time: Expected Current:
Prepare Samples
Denaturing Samples: Add 50 mL 20X NuPAGE Tris-Acetate SDS Running Buffer to 950 mL deionized water. Native Samples: Add 100 mL 10X TrisGlycine Native Running Buffer to 900 mL deionized water.
Reagent Reduced Sample Sample x μL Tris-Glycine SDS Sample Buffer (2X) 5 μL NuPAGE Reducing Agent (10X) 1 μL Deionized Water to 4 μL Total Volume 10 μL
Non-reduced Sample x μL 5 μL -to 5 μL 10 μL
Heat samples at 85˚C for 2 minutes.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Prepare 1X Buffer
Load Buffer
Fill the Upper (200 mL) and Lower (600 mL) Buffer Chambers with the appropriate 1X Running Buffer. For reduced samples, use 200 mL 1X Running Buffer with 500 μL NuPAGE Antioxidant in the Upper Buffer Chamber.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Load Buffer
Fill the Upper Buffer Chamber with 200 mL and the Lower Buffer Chamber with 600 mL of 1X Tris-Glycine SDS Running Buffer.
Conditions
Run
Voltage: Run Time: Expected Current:
Blot Gel
Run Conditions
Voltage: Run Time: Expected Current:
150 V constant 1 hour (Denaturing gel), 2–3 hours (Native gel) 40–55 mA/gel (start); 25–40 mA/gel (end) for denaturing gel 18 mA/gel (start); 7 mA/gel (end) for native gel
Conditions
Add 100 mL 10X Novex Tris-Glycine SDS Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine SDS Running Buffer.
125 V constant 90 minutes (dependent on gel percentage) 30–40 mA/gel (start); 8–12 mA/gel (end)
125 V constant 1–12 hours 6–12 mA/gel (start); 3–6 mA/gel (end)
For blotting denaturing and native gels, use 1X Tris-Glycine Transfer Buffer with 20% methanol. Perform blotting at 25 V constant for 1–2 hours using the XCell II Blot Module. The expected start current is 100 mA.
Intended Use: For research use only. Not for human or animal therapeutic or diagnostic use.
Appendix
78
Protein gel electrophoresis technical handbook
Appendix
79
Protocol quick reference QUICK REFERENCE
QUICK REFERENCE
Tris-Glycine Midi Gels
Non-denaturing (Native) Electrophoresis
Instructions for electrophoresis using the XCell4 SureLock Midi-Cell are described below.
Prepare Samples
Reagent
Reduced sample
Sample Tris-Glycine SDS Sample Buffer (2X) NuPAGE Reducing Agent (10X) Deionized Water
x µL 5 µL 1 µL to 10 µL final
Non-reduced sample x µL 5 µL — to 10 µL final
Heat samples at 85˚C for 2 minutes.
Prepare 1X Buffer
Add 100 mL 10X Tris-Glycine SDS Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine SDS Running Buffer.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Add Buffer
Fill each Upper Buffer Chamber with 175 mL 1X Tris-Glycine SDS Running Buffer. Fill the Lower Buffer Chamber up to the fill line mark with 1X Tris-Glycine SDS Running Buffer.
Run Conditions
Voltage: Run Time: Expected Current:
Prepare Samples
Reagent Sample Tris-Glycine Native Sample Buffer (2X) Deionized Water
Native Sample x µL 5 µL to 10 µL final
Do not heat native samples.
Prepare 1X Buffer
Add 100 mL 10X Tris-Glycine SDS Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine SDS Running Buffer.
Load Sample
Load the appropriate concentration of your protein sample on the gel.
Add Buffer
Fill each Upper Buffer Chamber with 175 mL of 1X Tris-Glycine Native Running Buffer. Fill the Lower Buffer Chamber up to the fill line mark with 1X TrisGlycine Native Running Buffer.
Run Conditions
Voltage: Run Time: Expected Current:
125 V constant 1–12 hours 35–40 mA/gel (start); 15–20 mA/gel
Tricine Gels
Tricine Gels
Instructions are provided below for electrophoresis of Tricine Gels using the XCell SureLock Mini-Cell.
Blotting Conditions
For blotting Tricine gels, use 1X Tris-Glycine Transfer Buffer with 20% methanol. Perform transfer with nitrocellulose or PVDF membranes at 25 V constant for 1–2 hours using the XCell II Blot Module. The expected start current is 100 mA.
Alternate Transfer Buffers
The Tris-Glycine Transfer Buffer interferes with protein sequencing. If you are performing protein sequencing, use 1X NuPAGE Transfer Buffer or 0.5X TBE Transfer Buffer for blotting. The NuPAGE Transfer Buffer protects against modification of the amino acid side chains and is compatible with N-terminal protein sequencing using Edman degradation.
Prepare Samples
Reagent Reduced Sampl e Sam pl e x μL Tricine SDS Sample Buffer (2X) 5 μL NuPAGE Reducing Agent (10X) 1 μL Deionized Water to 4 μL Tot al Vol u m e 10 μ L
Non- r educed Sampl e x μL 5 μL -to 5 μL 10 μ L
Heat samples at 85˚C for 2 minutes.
Prepare 1X Buffer
Add 100 mL 10X Novex Tricine SDS Running Buffer to 900 mL deionized water to prepare 1X Tricine SDS Running Buffer.
Load Sample Load the appropriate concentration of your protein sample on the gel. Load Buffer Fill the Upper Buffer Chamber with 200 mL and the Lower Buffer Chamber
125 V constant 105 min (dependent on gel percentage) 40–50 mA/gel (start); 20–25 mA/gel (end)
with 600 mL of 1X Tricine SDS Running Buffer.
Run Conditions
For Research Use Only. Not for use in diagnostic procedures.
Voltage: Run Time: Expected Current:
125 V constant 90 minutes (dependent on gel percentage) 80 mA/gel (start); 40 mA/gel (end)
For research use only. Not for human or animal therapeutic or diagnostic use.
QUICK REFERENCE
QUICK REFERENCE
Staining Protocol
NativePAGE Bis-Tris Gels ™
Instructions are provided below for electrophoresis of NativePAGE™ Bis-Tris Gels using the XCell SureLock Mini-Cell.
Prepare Samples
Reagent Sample NativePAGE™ Sample Buffer (4X) NativePAGE™ 5% G-250 Additive Deionized Water
Sample with detergent x μL 2.5 μL 0.25–1 μL* to 10 μL
Detergent-free sample x μL 2.5 μL — to 10 μL
Do not heat samples for native gel electrophoresis. *Ensure the final G-250 concentration is ¼th the detergent concentration.
Prepare 1X Running Buffer
1X NativePAGE Anode Buffer: Add 50 mL 20X NativePAGE Running Buffer to 950 mL deionized water. 1X NativePAGE™ Cathode Buffer: Add 50 mL 20X NativePAGE™ Running Buffer and 50 mL 20X NativePAGE™ Cathode Additive to 900 mL deionized water. ™
™
Load Sample Fill wells with 1X NativePAGE™ Cathode Buffer and load samples prior to filling
the cathode chamber. Load an appropriate amount of your sample on the gel.
Add Buffer Run Conditions
A quick staining protocol for NativePAGE Gels using the Coomassie G-250 from the sample additive is described below. The total staining time is ~2–3 hours. Sensitivity is ~60 ng BSA. Step
1 2
Action
Time
Place the gel in 100 mL fixing solution (40% methanol, 10% acetic acid) and microwave on high (950–1100 watts).
45 seconds
Shake the gel on an orbital shaker.
15 minutes
3
Discard fixing solution.
4
Place the gel in 100 mL destain solution (8% acetic acid) and microwave on high (950–1100 watts).
5
Shake the gel on an orbital shaker until the desired background is obtained.
IEF Gels
IEF Gels
Instructions are provided below for electrophoresis of IEF Gels using the XCell SureLock Mini-Cell.
Prepare for 1. Stain and destain the IEF gel. Incubate the IEF gel in 100 mL 20% ethanol 2D SDS/ for 10 minutes. PAGE 2. Cut out the desired lane (strip) from the gel for transfer to a SDS gel.
Prepare Samples
— 45 seconds
Reagent Sample IEF Sample Buffer pH 3–10 or pH 3–7 (2X) Deionized Water
Prepare 1X Buffer
1X IEF Anode Buffer: Add 20 mL 50X IEF Anode Buffer to 980 mL deionized water. Chill to 4°C to 10°C. 1X IEF Cathode Buffer: Add 20 mL IEF Cathode Buffer pH 3–10 (10X) or pH 3–7 (10X) to 180 mL deionized water. Chill to 4°C to 10°C.
Load Sample
Load the appropriate concentration and volume of your protein on the gel.
Add Buffer
Fill the Upper Buffer Chamber with chilled 200 mL 1X IEF Cathode Buffer and the Lower Buffer Chamber with chilled 600 mL 1X IEF Anode Buffer.
Run
Voltage: Expected Current:
—
Conditions
100 V constant for 1 hour 200 V constant for 1 hour 500 V constant for 30 minutes 7 mA/gel (start); 5 mA/gel (end)
Fill the Upper Buffer Chamber with ~200 mL 1X NativePAGE™ Cathode Buffer. Fill the Lower Buffer Chamber with ~550 mL 1X NativePAGE™ Anode Buffer.
Stain Gel
Voltage: Run Time: Expected Current:
For Research Use Only. Not for use in diagnostic procedures.
150 V constant 90–115 min (3–12% gel); 105–120 min (4–16% gel) 12–16 mA/gel (start); 2–4 mA/gel (end)
Sample x μL 5 μL to 10 μL final
Fix the IEF gel in 12% TCA or 12% TCA containing 3.5% sulfosalicylic acid for 30 minutes. Stain the IEF gel with method of choice.
3. Incubate the gel strip in 2 mL 2X SDS sample buffer and 0.5 mL ethanol for 3–5 minutes. Aspirate the sample buffer and rinse the gel strip with 1X SDS Running Buffer. 4. Fill the SDS gel cassette with 1X SDS Running Buffer. 5. Trim the IEF gel strip to a length of 5.8–5.9 cm. 6a. Transfer the gel strip into a 1.0 mm SDS gel by sliding the strip into the 2D-well using a gel loading tip. Avoid trapping air-bubbles between the strip and the SDS gel. Wet a piece of thick filter paper (5.8 cm × 4 cm) in SDS Running Buffer and insert the long edge of the paper into the 2D-well to push the gel strip into with the SDS gel. 6b. If transferring the gel strip into a 1.5 mm SDS gel, wet 2 pieces of thin filter paper (5.8 cm × 4 cm) in 1X SDS Running Buffer. Sandwich the gel strip between the two filter papers along the long edge with the edge of the strip protruding ~0.5 mm beyond the paper. Insert the sandwich into the 2D-well of the SDS gel without trapping air bubbles and push the strip down so it is in with the SDS gel. 7. Insert gel into the mini-cell, fill the buffer chambers with 1X SDS Running Buffer, and perform SDS-PAGE. 8. After the dye front has moved into the stacking gel (~10 minutes), disconnect power, remove the paper, and resume electrophoresis.
For Research Use Only. Not for use in diagnostic procedures.
Appendix
80
Protein gel electrophoresis technical handbook
Appendix
81
Ordering information QUICK REFERENCE
Zymogram Gels
Zymogram Gels
Instructions are provided below for electrophoresis of Zymogram Gels using the XCell SureLock Mini-Cell.
Develop Gel
Prepare Samples
Prepare 1X Buffer
Reagent Sample Sample x μL Tris-Glycine SDS Sample Buffer (2X) 5 μL Deionized Water to 10 μL final Do not heat or reduce samples for Zymogram gels. 1X Tris-Glycine SDS Running Buffer: Add 100 mL 10X Tris-Glycine SDS Running Buffer to 900 mL deionized water.
Load Sample
Load the appropriate concentration and volume of your protein on the gel.
Add Buffer
Fill the Upper Buffer Chamber with 200 mL, and the Lower Buffer Chamber with 600 mL of 1X Tris-Glycine SDS Running Buffer.
Run Conditions
Voltage: Run Time: Expected Current:
125 V constant 90 minutes (dependent on gel percentage) 30–40 mA/gel (start); 8–12 mA/gel (end)
For Research Use Only. Not for use in diagnostic procedures.
Stain Gel
Sensitivity Level
Product
1. Dilute Zymogram Renaturing Buffer (10X) and Zymogram Developing Buffer (10X) 1:9 with deionized water. Prepare 100 mL of each 1X buffer per 1–2 mini-gels. 2. After electrophoresis, incubate the gel in 1X Zymogram Renaturing Buffer for 30 minutes at room temperature with gentle agitation. 3. Decant Zymogram Renaturing Buffer and add 1X Zymogram Developing Buffer to the gel. 4. Equilibrate the gel for 30 minutes at room temperature with gentle agitation. 5. Decant buffer and add fresh 1X Zymogram Developing Buffer to the gel. 6. Incubate the gel at 37°C for at least 4 hours or overnight for maximum sensitivity. Optimize results empirically by varying the sample load or incubation time. Zymogram (Blue Casein) 4–16% gels do not require staining. For non-prestained Zymogram gels, stain the gels with Colloidal Blue Staining Kit or the SimplyBlue Safestain. Areas of protease activity appear as clear bands against a dark background. 10% Zymogram (Gelatin) Gel: 12% Zymogram (Casein) Gel: 4–16% Zymogram (Blue Casein) Gel:
10-6 units of collagenase 7 × 10-4 units of trypsin 1.5 × 10-3 units of trypsin
Quantity
Cat. No.
Product
Quantity
Cat. No.
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 9-well
1 box
NP0307BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.5 mm, 10-well
1 box
NP0315BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.5 mm, 15-well
1 box
NP0316BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 1-well
1 box
NP0344BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 10-well
10 gels
NP0341BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 10-well
2 gels
NP0341PK2
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 12-well
10 gels
NP0342BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 12-well
2 gels
NP0342PK2
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 15-well
1 box
NP0343BOX
NuPAGE™ Novex™ 12% Bis-Tris Protein Gels, 1.0 mm, 17-well
1 box
NP0349BOX
1 box
NP0324BOX
NW3010
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 1-well
10 gels
NP0321BOX
NW3012
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 10-well NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 10-well
2 gels
NP0321PK2
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 12-well
10 gels
NP0322BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 12-well
2 gels
NP0322PK2
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 15-well
10 gels
NP0323BOX
Select precast gel Bolt Bis-Tris Plus Gels Bolt 8% Bis-Tris Plus Gels, 10-well
1 box
NW00080BOX
Bolt™ 8% Bis-Tris Plus Gels, 12-well
1 box
NW00082BOX
Bolt™ 8% Bis-Tris Plus Gels, 15-well
1 box
NW00085BOX
Bolt™ 8% Bis-Tris Plus Gels, 17-well
1 box
NW00087BOX
Bolt™ 10% Bis-Tris Plus Gels, 10-well
1 box
NW00100BOX
Bolt 10% Bis-Tris Plus Gels, 12-well
1 box
NW00102BOX
Bolt™ 10% Bis-Tris Plus Gels, 15-well
1 box
NW00105BOX
Bolt 10% Bis-Tris Plus Gels, 17-well
1 box
NW00107BOX
Bolt 12% Bis-Tris Plus Gels, 10-well
1 box
NW00120BOX
Bolt™ 12% Bis-Tris Plus Gels, 12-well
1 box
NW00122BOX
Bolt 12% Bis-Tris Plus Gels, 15-well
1 box
NW00125BOX
Bolt™ 12% Bis-Tris Plus Gels, 17-well
1 box
NW00127BOX
Bolt 4–12% Bis-Tris Plus Gels, 10-well
1 box
NW04120BOX
Bolt 4–12% Bis-Tris Plus Gels, 12-well
1 box
NW04122BOX
Bolt™ 4–12% Bis-Tris Plus Gels, 15-well
1 box
NW04125BOX
Bolt 4–12% Bis-Tris Plus Gels, 17-well
1 box
NW04127BOX
Bolt™ Empty Mini Gel Cassettes
20 cassettes NW2010
Bolt Empty Mini Gel Cassette Combs, 10-well
20 combs
Bolt™ Empty Mini Gel Cassette Combs, 12-well
20 combs
Bolt Welcome Pack B, 4–12%, 15-well
1 kit**
NW0412B
Bolt™ Welcome Pack A, 4–12%, 10-well
1 kit**
NW0412A
™
™
™
™
™
™
™
™
™
™
One box contains 10 gels. ** B olt Welcome Pack kit includes Mini Gel Tank, 2 boxes of Bolt 4–12% Gels (10-well/15-well), Bolt MES Running Buffer (20X), Bolt LDS Sample Buffer (4X), Bolt Sample Reducing Agent (10X) and SeeBlue™ Plus2 Prestained Standard
NuPAGE Bis-Tris Mini Gels (8 cm x 8 cm) NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 1-well
1 box
NP0304BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 15-well
2 gels
NP0323PK2
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 10-well
10 gels
NP0301BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 17-well
10 gels
NP0329BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 10-well
2 gels
NP0301PK2
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 17-well
2 gels
NP0329PK2
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 12-well
10 gels
NP0302BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.0 mm, 9-well
1 box
NP0327BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 12-well
2 gels
NP0302PK2
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.5 mm, 10-well
10 gels
NP0335BOX
NuPAGE™ Novex™ 10% Bis-Tris Protein Gels, 1.0 mm, 15-well
1 box
NP0303BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.5 mm, 10-well
2 gels
NP0335PK2
Appendix
82
Protein gel electrophoresis technical handbook
Appendix
83
Ordering information Product
Quantity
Cat. No.
Product
Quantity
Cat. No.
Product
Quantity
Cat. No.
Product
Quantity
Cat. No.
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.5 mm, 15-well
10 gels
NP0336BOX
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 10-well
2 gels
EA0375PK2
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6078BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6505BOX
NuPAGE™ Novex™ 4–12% Bis-Tris Protein Gels, 1.5 mm, 15-well
2 gels
NP0336PK2
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 12-well
10 gels
EA03752BOX
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC60785BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC65052BOX
2 gels
EA03752PK2
Novex™ 10–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6135BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC65055BOX
WG1201BOX
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 12-well
1 box
EA03755BOX
Novex™ 10–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC61352BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6508BOX
WG1201A
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 15-well
1 box
EA0378BOX
Novex™ 10–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC61355BOX
Novex™ 18% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC65085BOX
WG1202BOX
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.5 mm, 10-well
1 box
EA03785BOX
Novex™ 10–20% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC61385BOX
Novex™ 4% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6055BOX
WG1202A
NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.5 mm, 15-well
1 box
EA0355BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.0 mm, 1-well
1 box
EC6001BOX
Novex™ 4% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60552BOX
WG1203BOX
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.0 mm, 10-well
1 box
EA03552BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6005BOX
Novex™ 4% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6058BOX
WG1203A
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.0 mm, 12-well
1 box
EA03555BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60052BOX
Novex™ 4% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC60585BOX
WG1401BOX
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.0 mm, 15-well
1 box
EA0358BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC60055BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6035BOX
WG1401A
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.5 mm, 10-well
1 box
EA03585BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6008BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60352BOX
WG1402BOX
NuPAGE™ Novex™ 7% Tris-Acetate Protein Gels, 1.5 mm, 15-well
1 box
EC60085BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC60355BOX
WG1601BOX
Novex™ 12% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC6485BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6038BOX
WG1601A
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC64852BOX
Novex™ 4–12% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC60385BOX
WG1602BOX
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC64855BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 1-well
1 box
EC6021BOX
WG1602A
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC6488BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6025BOX
WG1603BOX
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC64885BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60252BOX
WG1603A
Novex™ 14% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well Novex™ 16% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6495BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC60255BOX
NuPAGE Bis-Tris Midi Gels (8 cm x 13 cm) NuPAGE Novex 10% Bis-Tris Midi Protein Gels, 12+2-well
1 box
NuPAGE Novex 10% Bis-Tris Midi Protein Gels, 12+2-well, w/adapters
1 box
NuPAGE™ Novex™ 10% Bis-Tris Midi Protein Gels, 20-well
1 box
NuPAGE Novex 10% Bis-Tris Midi Protein Gels, 20-well, w/adapters
1 box
NuPAGE Novex 10% Bis-Tris Midi Protein Gels, 26-well
1 box
NuPAGE™ Novex™ 10% Bis-Tris Midi Protein Gels, 26-well, w/adapters
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 12+2-well
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 12+2-well, w/adapters
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 20-well
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 20-well, w/adapters
1 box
WG1402A
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 12+2W
1 box
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 26-well
1 box
WG1403BOX
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 12+2W, w/adapters
1 box
™
NuPAGE Novex 4–12% Bis-Tris Midi Protein Gels, 26-well, w/adapters
1 box
WG1403A
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 20W
1 box
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 12+2-well
1 box
WG1001BOX
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 20W, w/adapters
1 box
™
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 12+2-well, w/adapters
1 box
WG1001A
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 26W
1 box
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 20-well
1 box
WG1002BOX
NuPAGE Novex 3–8% Tris-Acetate Midi Protein Gels, 26W, w/adapters
1 box
NuPAGE™ Novex™ 8% Bis-Tris Midi Protein Gels, 20-well, w/adapters
1 box
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 26-well
1 box
NuPAGE Novex 8% Bis-Tris Midi Protein Gels, 26-well, w/adapters
1 box
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
™
NuPAGE Tris-Acetate Midi Gels (8 cm x 8 cm)
10 gels
™
™
™
™
™
™
™
™
™
™
™
WG1002A
Novex Tris-Glycine Mini Gels (8 cm x 8 cm) 1 box
EC6075BOX
Novex™ 16% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC64952BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.0 mm, 9-well
1 box
EC60249BOX
WG1003BOX
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
3 boxes
EC6075BX30
Novex™ 16% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC64955BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6028BOX
WG1003A
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well - Value Pack Novex™ 10% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60752BOX
Novex™ 16% Tris-Glycine Mini Protein Gels, 1.5 mm, 10-well
1 box
EC6498BOX
Novex™ 4–20% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC60285BOX
Novex™ 10% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
1 box
EC60755BOX
Novex™ 16% Tris-Glycine Mini Protein Gels, 1.5 mm, 15-well
1 box
EC64985BOX
Novex™ 6% Tris-Glycine Mini Protein Gels, 1.0 mm, 10-well
1 box
EC6065BOX
NuPAGE Tris-Acetate Mini Gels (8 cm x 8 cm) NuPAGE™ Novex™ 3–8% Tris-Acetate Protein Gels, 1.0 mm, 10-well
™
EA0375BOX
Appendix
84
Protein gel electrophoresis technical handbook
Appendix
85
Ordering information Product
Quantity
Cat. No.
Product
Quantity
Cat. No.
Product
Novex™ 6% Tris-Glycine Mini Protein Gels, 1.0 mm, 12-well
1 box
EC60652BOX
Novex™ 4–20% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
WT4201A
Novex Tricine Gels 1 box
EC6675BOX
Pierce™ SDS-PAGE Sample Prep Kit
1 box
Novex 4–20% Tris-Glycine Midi Protein Gels, 20-well
1 box
WT4202BOX
50 reactions
89888
Novex 6% Tris-Glycine Mini Protein Gels, 1.0 mm, 15-well
Novex 10% Tricine Protein Gels, 1.0 mm, 10-well
1 box
EC66752BOX
Bolt Transfer Buffer (20X)
125 mL
BT0006
1 box
WT4202A
Bolt Transfer Buffer (20X)
1L
BT00061
WT0101BOX
Novex 4–20% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
Novex™ 10% Tricine Protein Gels, 1.0 mm, 12-well
1 box
EC6695BOX
4X Bolt LDS Sample Buffer
10 mL
B0007
1 box
WT4203BOX
WT0101A
Novex™ 4–20% Tris-Glycine Midi Protein Gels, 26-well
Novex™ 16% Tricine Protein Gels, 1.0 mm, 10-well
1 box
EC66952BOX
20X Bolt MES SDS Running Buffer
500 mL
B0002
1 box
WT4203A
WT0102BOX
Novex™ 4–20% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
Novex™ 16% Tricine Protein Gels, 1.0 mm, 12-well
1 box
WT0102A
Novex™ 8% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
WT0103BOX
Novex™ 8% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
WT0103A
Novex™ 8% Tris-Glycine Midi Protein Gels, 20-well
1 box
WT0121BOX
Novex™ 8% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
WT0083BOX
Novex™ pH 3–7 IEF Protein Gels, 1.0 mm, 12-well
5 gels⁄box
WT0121A
Novex™ 8% Tris-Glycine Midi Protein Gels, 26-well
1 box
WT0083A
WT0122BOX
Novex™ 8% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
Novex™ pH 3–7 IEF Protein Gels, 1.0 mm, 10-well
1 box
WT8161BOX
WT0122A
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 12+2-well
Novex™ pH 3–10 IEF Protein Gels, 1.0 mm, 10-well
1 box
WT8161A
Novex Zymogram Gels
WT0123BOX
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
WT0123A
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 20-well
1 box
WT4121BOX
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
WT4121A
Novex™ 8–16% Tris-Glycine Midi Protein Gels, 26-well Novex™ 8–16% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
1 box
™
EC60655BOX
Novex Tris-Glycine Midi Gels (8 cm x 13 cm) Novex™ 10% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
Novex™ 10% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
Novex™ 10% Tris-Glycine Midi Protein Gels, 20-well
1 box
Novex 10% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
Novex™ 10% Tris-Glycine Midi Protein Gels, 26-well
1 box
Novex™ 10% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 20-well
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 26-well
1 box
Novex 12% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
1 box
Novex 4–12% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
Novex 4–12% Tris-Glycine Midi Protein Gels, 12+2-well, w/adapters
1 box
™
™
™
™
™
™
™
™
™
™
™
™
WT4122BOX
Novex™ 4–12% Tris-Glycine Midi Protein Gels, 20-well, w/adapters
1 box
WT4122A
NativePAGE™ Novex™ 3–12% Bis-Tris Protein Gels, 1.0 mm, 10-well
1 box
Novex™ 4–12% Tris-Glycine Midi Protein Gels, 26-well
1 box
WT4123BOX
NativePAGE™ Novex™ 3–12% Bis-Tris Protein Gels, 1.0 mm, 15-well
1 box
Novex 4–12% Tris-Glycine Midi Protein Gels, 26-well, w/adapters
1 box
NativePAGE Novex 4–16% Bis-Tris Protein Gels, 1.0 mm, 10-well
1 box
Novex™ 4–20% Tris-Glycine Midi Protein Gels, 12+2-well
1 box
NativePAGE™ Novex™ 4–16% Bis-Tris Protein Gels, 1.0 mm, 15-well
1 box
WT4123A WT4201BOX
Quantity
Cat. No.
EC66955BOX
20X Bolt MES SDS Running Buffer
5L
B0002-02
20X Bolt MOPS SDS Running Buffer
500 mL
B0001
20X Bolt MOPS SDS Running Buffer
5L
B0001-02
1 box
WT0081BOX
Novex 10–20% Tricine Protein Gels, 1.0 mm, 10-well
1 box
EC6625BOX
Bolt Antioxidant
15 mL
BT0005
WT0081A
LA0041
1 box
EC66252BOX
NuPAGE Tris-Acetate SDS Running Buffer (20X)
500 mL
WT0082BOX
Novex™ 10–20% Tricine Protein Gels, 1.0 mm, 12-well
NP0001
1 box
EC66255BOX
NuPAGE™ MOPS SDS Running Buffer (20X)
500 mL
WT0082A
Novex™ 10–20% Tricine Protein Gels, 1.0 mm, 15-well
NuPAGE™ MOPS SDS Running Buffer (20X)
5L
NP000102
EC66452BOX
NuPAGE™ MES SDS Running Buffer (20X)
5L
NP000202
5 gels⁄box
EC6645BOX
NuPAGE™ MES SDS Running Buffer (20X)
500 mL
NP0002
5 gels⁄box
EC6655BOX
Novex Tris-Glycine SDS Running Buffer (10X)
4x1L
LC26754 LC2675
1 box
EC64052BOX
Novex Tris-Glycine SDS Running Buffer (10X)
500 mL
WT8162BOX
Novex™ 12% Zymogram (Casein) Protein Gels, 1.0 mm, 12-well
LC26755
1 box
EC6415BOX
Novex Tris-Glycine SDS Running Buffer (10X)
5L
WT8162A
Novex™ 4–16% Zymogram (Blue Casein) Protein Gels, 1.0 mm, 10-well
LC1675
1 box
EC6405BOX
Novex™ Tricine SDS Running Buffer (10X)
500 mL
WT8163BOX
Novex™ 12% Zymogram (Casein) Protein Gels, 1.0 mm, 10-well
NuPAGE™ LDS Sample Buffer (4X)
10 mL
NP0007
1 box
EC61755BOX
WT8163A
Novex™ 10% Zymogram (Gelatin) Protein Gels, 1.0 mm, 15-well
Novex Tricine SDS Sample Buffer (2X)
20 mL
LC1676
20 mL
LC2676
™
Novex™ 10% Zymogram (Gelatin) Protein Gels, 1.0 mm, 12-well
1 box
EC61752BOX
Novex Tris-Glycine SDS Sample Buffer (2X)
BN1001BOX
Novex™ 10% Zymogram (Gelatin) Protein Gels, 1.0 mm, 10-well
1 box
EC6175BOX
Novex™ Tris-Glycine Transfer Buffer (25X)
500 mL
LC3675
BN1003BOX
E-PAGE™ High Throughput Gel System
NuPAGE™ Transfer Buffer (20X)
125 mL
NP0006
E-PAGE™ 8% Protein Gels, 48-well
EP04808
NuPAGE Transfer Buffer (20X)
1L
NP00061
EH03
NuPAGE Antioxidant
15 mL
NP0005
EPBUF01
Novex Tris-Glycine SDS Buffer Kit
1 kit
LC2677
NuPAGE MOPS SDS Buffer Kit (for Bis-Tris Gels)
1 kit
NP0050
NuPAGE™ MES SDS Buffer Kit (for Bis-Tris Gels)
1 kit
NP0060
NativePAGE Gels
™
Product
Prepare samples and select buffers: SDS-PAGE
Novex IEF Gels
1 box
™
Cat. No.
Novex™ 16% Tricine Protein Gels, 1.0 mm, 15-well ™
Novex™ 4–12% Tris-Glycine Midi Protein Gels, 20-well
™
Quantity
BN1002BOX BN1004BOX
E-Holder™ Platform E-PAGE™ Loading Buffer 1
8 gels 2 units 4.5 mL
E-PAGE™ 6% Protein Gels, 96-well
8 gels
EP09606
Daughter E-Base™ Device
1 unit
EBD03
Mother E-Base Device
1 unit
EBM03
™
™
™
™
™
Appendix
86
Protein gel electrophoresis technical handbook
Appendix
87
Ordering information Product
Quantity
Cat. No.
Product
NuPAGE™ Tris-Acetate SDS Buffer Kit (for Tris-Acetate gels), Contains 1 ea. LA0041, NP0004, NP0005, NP0007
1 kit
LA0050
Select Protein Standards: Unstained
Novex™ Tricine SDS Buffer Kit, includes LC1676 & LC1675
1 kit
Pierce LDS Sample Buffer, NonReducing (4X) Pierce Lane Marker Non-Reducing Sample Buffer
5 mL 5 mL
LC1677 84788 39001
Pierce 10X Tris-Glycine SDS Buffer
1L
28362
BupH Tris-Glycine-SDS Buffer Packs
40 packs
28378
™
Native Electrophoresis Novex Tris-Glycine Native Running Buffer (10X)
500 mL
LC2672
Novex Tris-Glycine Native Sample Buffer (2X)
20 mL
LC2673
NativePAGE™ Running Buffer (20X)
1L
BN2001
NativePAGE™ Running Buffer Kit
1 kit
BN2007
NativePAGE™ Cathode Buffer Additive (20X)
250 mL
BN2002
NativePAGE™ Sample Buffer (4X)
Quantity
Cat. No.
PageRuler™ Unstained Low Range Protein Ladder
2 x 250 µL
26632
PageRuler™ Unstained Protein Ladder
2 x 250 µL
26614
NativeMark Unstained Protein Standard
5 x 50 µL
LC0725
Prestained
BN2003
Mini Gel Tank
1 unit
A25977
PageBlue Protein Staining Solution
1L
24620
XCell SureLock Mini-Cell
1 unit
EI0001
SimplyBlue SafeStain
1L
LC6060
XCell4 SureLock™ Midi-Cell
1 each
WR0100
SimplyBlue SafeStain
3.5 L
LC6065
PowerEase™ 90W Power Supply (115 VAC)
1 each
PS0090
Imperial Protein Stain
1L
24615
Imperial Protein Stain
3x1L
24617
PowerEase™ 90W Power Supply (230 VAC)
1 each
PS0091
Pierce™ Silver Stain Kit
1L
24612
SilverXpress™ Silver Staining Kit
1 kit*
LC6100
Pierce™ Silver Stain for Mass Spectrometry
1L
24600
SYPRO™ Orange Protein Gel Stain
500 µL
S-6650
SYPRO™ Orange Protein Gel Stain
10 x 50 µL
S-6651
™
™
2 x 250 µL
26619
PowerEase™ 300W Power Supply (115 VAC)
1 each
PS0300
PageRuler™ Plus Prestained Protein Ladder
10 x 250 µL
26620
PowerEase™ 300W Power Supply (230 VAC)
1 each
PS0301
Spectra™ Multicolor Broad Range Protein Ladder
2 x 250 µL
26634
Spectra™ Multicolor Broad Range Protein Ladder
10 x 250 µL
26623
HiMark Prestained Protein Standard
250 µL
LC5699
SYPRO™ Red Protein Gel Stain
500 µL
S-6653
Spectra Multicolor High Range Protein Ladder
2 x 250 µL
26625
SYPRO™ Red Protein Gel Stain
10 x 50 µL
S-6654
SYPRO Ruby Protein Gel Stain
1L
S-12000
SYPRO Ruby Protein Gel Stain
200 mL
S-12001
SYPRO Ruby Protein Gel Stain
5L
S-21900
Pro-Q Emerald 488 Glycoprotein Gel Stain Kit
1 kit
P-21875
Pro-Q™ Diamond Phosophoprotein Gel Stain Kit
1L
P-33300
Pro-Q™ Diamond Phosophoprotein Gel Stain Kit
200 mL
P-33301
Pro-Q™ Diamond Phosophoprotein Gel Stain Kit
5L
P-33302
Pierce Power Stainer
1 unit
22833
Pierce Power Stainer Welcome Pack
1 unit
22833SPCL*
™
™
Silver stains
*1 kit contains sufficient reagents to stain 25 mini gels
Fluorescent and specialty stains
™
™
MagicMark™ XP Western Protein Standard
250 µL
LC5602
DDM (n-dodecyl β-D-maltoside) (10%)
1 mL
BN2005
Specialty
1 mL
BN2006
PageRuler Prestained NIR Protein Ladder
2 x 250 µL
500 mL
LC2671
BenchMark™ Fluorescent Protein Standard
125 µL
LC2670
BenchMark His-tagged Protein Standard
125 µL
LC5606
IEF Marker 3–10
500 µL
39212-01
™
™
IEF
Coomassie stains
PageRuler™ Plus Prestained Protein Ladder
BN2008
500 mL
Electrophoresis chamber systems and power supplies
26617
1 kit
Novex™ Zymogram Renaturing Buffer (10X)
50 µL
LC5603
26635 LC5928
™
™
Pierce Power Stainer
™
Novex™ IEF Anode Buffer (50X)
100 mL
LC5300
Novex™ IEF Cathode Buffer pH 3-10 (10X) 125 mL
LC5310
Novex™ IEF Cathode Buffer pH 3-7 (10X)
125 mL
LC5370
Novex pH 3-10 IEF Buffer Kit, Includes LC5300, LC5310, LC5311
1 kit
LC5317
Novex™ pH 3-7 IEF Buffer Kit, Includes LC5300, LC5370, LC5371
1 kit
LC5377
Novex™ IEF Sample Buffer pH 3-10 (2X)
25 mL
LC5311
Novex IEF Sample Buffer pH 3-7 (2X)
25 mL
LC5371
™
™
Cat. No.
10 x 250 µL
NativePAGE Sample Prep Kit
Novex™ Zymogram Developing Buffer (10X)
Quantity
PageRuler™ Prestained Protein Ladder
MagicMark™ XP Western Protein Standard
Zymography
Product
26616
0.5 mL
Digitonin (5%)
Cat. No.
2 x 250 µL
NativePAGE™ 5% G-250 Sample Additive ™
BN2004
Quantity
PageRuler™ Prestained Protein Ladder
Western 10 mL
Product
*Welcome pack includes Pierce Power Station, Pierce Power Stain Cassette, Western Blot Roller, Power Cord with C/13 Connector, Quick Start Guide, Pierce Mini Gel Power Staining Kit
Appendix Appendix
Crossword puzzle challenge To participate in the crossword puzzle challenge, Separate Crossword
go to thermofisher.com/pagecrossword Complete the crossword below 1
2
3 4 5
6
7 8
9
10 11
12
13
14
15
Across
Created on TheTeachersCorner.net Crossword Maker
Xcell4SureLock
DownHermannSchagger UlrichLaemmli ArneTiselius
SheldonEngelhorn
5. Can you name one of the scientists who developed 1. Which tank is compatible with >180 mini gels? pg. 52 BisTris MagicMark PowerStainer Bolt minimizes Minigeltank HarrySvennsonRilbe blue native polyacrylamide gel electrophoresis? 2. Which gel chemistry protein degradation? pg. 9 pg. 17Unstained 6. Which tank is compatible with midigels? pg. 56 3. Who first published SDS-PAGE as a method for the Prestained PowerEase NuPAGE 9. Which ladder can be used for accurate molecular analysis of cleavage of structural proteins in weight estimation directly on western blots? pg. 46 bacteriophage? pg. 7 10. Can you name one of the scientists who filed a 4. Can you name one of the scientists who first patent for the netural-pH Bis-Tris system in 1996? pg. 11 described the theory of separation of amphoteric 12. Which gel has a unique wedge well design that proteins along a pH gradient in the 1960s? pg. 21 allows you to load 2x the sample volume? pg. 10 7. What power supply is available for use with the Mini 13. Which protein ladder would you use for Gel Tank? pg. 58 approximate determination of molecular weight? pg. 35 8. What is a fast alternative to traditional Coomassie 15. Who won the Nobel Prize for analysis of serum staining? pg. 72 proteins by electrophoresis in 1948? pg. 5 11. Which ladder would you use for precise determination of molecular weight? pg. 35 14. Which type of gel has been referenced in >20,000 publications? pg. 12
Learn more at thermofisher.com/invitrogen For Research Use Only. Not for use in diagnostic procedures. © 2015 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. Polaroid is a trademark of Polaroid Corporation. Triton is a trademark of Union Carbide Corporaton. CO015381 0715